All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Know AML webinar | Empowering patients to ask their physician the right questions during their AML journey
Know AML hosted a webinar for patients and healthcare professionals (HCPs) on April 23, 2025, titled ‘Mutation testing in AML: What you need to know’. Here, we share a discussion between Gail J. Roboz, Weill Cornell Medicine, New York, US, and Ralph Hills, Connecticut, US, where they considered the range of genetic mutations in AML and what they mean for treatments, and also how mutation testing methods used in the diagnosis of AML have changed in over the years.
Know AML webinar | Empowering patients to ask their physician the right questions
Know AML webinar | Empowering patients to ask their physician the right questions
They went on to debate challenges faced by patients during an AML diagnosis, including access to mutation testing and discussions between physicians and patients. They concluded by describing the importance of shared decision-making, and the role of physicians and patients in enabling clearer conversations about mutation testing in AML going forward.
Key points
The estimated number of new cases of AML in the US in 2024 was 20,800 – 1% of all new cancer cases in the US. Survival rates have improved steadily over the past 50 years.
Since 2017, many drugs designed to target specific mutations have been approved by the U.S. Food and Drug Administration (FDA) and the European Union (EU).
Patients face several challenges during the process of being diagnosed with AML, including lack of awareness of testing methods, the wait time for test results, and accessing appropriate testing methods; therefore, clear communication between physicians and patients is essential.
Several mutation testing methods are available, including conventional cytogenetics (karyotyping), fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), and next-generation sequencing (NGS), and these techniques continue to evolve (Figure 1).
Physicians play a vital role in guiding patients towards appropriate diagnostic tests needed to inform treatment decisions. Providing access to testing methods, whether as part of diagnosis and monitoring in clinics or through clinical trials, is important. For example, the US myeloMATCH trial uses NGS mutation testing to assign patients to treatments that target their specific mutations and then tests how well and safely these treatments work.
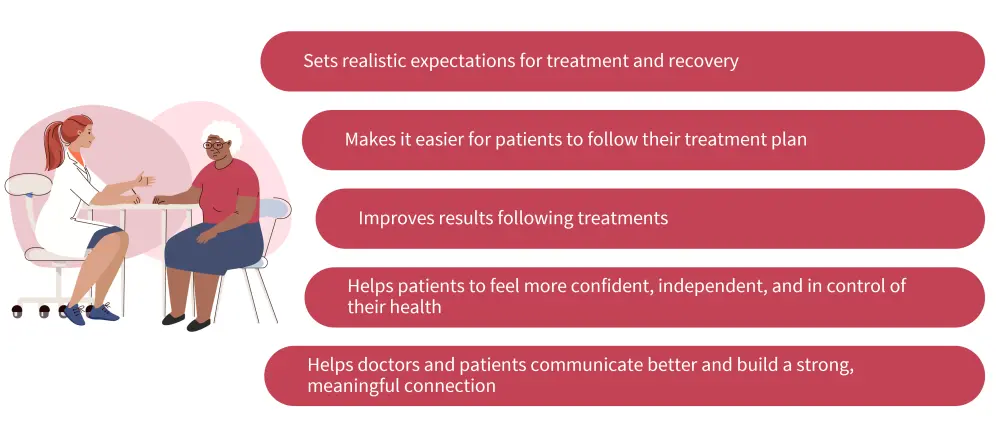
Shared decision-making between physicians and patients is crucial to set realistic expectations for treatment and recovery, improve results following treatments, and help patients to feel more confident, independent, and in control of their health (Figure 2).
Figure 1. Mutation testing methods: How far have we come?
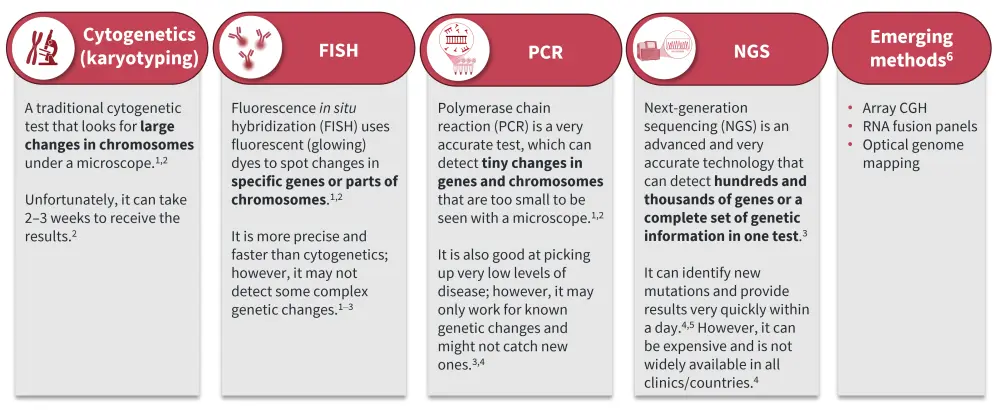
Figure 2. Importance of making decisions together7
This independent educational activity is supported by Thermo Fisher Scientific.
All content was developed independently by the faculty. The funder was allowed no influence on the content.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Charles Craddock
Charles Craddock Gail J. Roboz
Gail J. Roboz.webp&w=3840&q=75)
.webp&w=3840&q=75)
.webp&w=3840&q=75)

.webp&w=3840&q=75)