All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Know AML webinar | What are mutations, and why do they matter in AML?
Know AML hosted a webinar for patients and healthcare professionals (HCPs) on April 23, 2025, titled ‘Mutation testing in AML: What you need to know’. Here, we share a presentation by the chair, Charles Craddock, Queen Elizabeth Hospital Birmingham, UK, discussing mutations and why they matter in AML.
Know AML webinar | What are mutations, and why do they matter in AML?
Know AML webinar | What are mutations, and why do they matter in AML?
Craddock opened the session by introducing himself along with fellow speakers, Gail J. Roboz, Weill Cornell Medicine, New York, US, and Ralph Hills, Connecticut, US, who shared his personal journey living with AML.
Craddock began by outlining the discovery of DNA, the basics of genetics, and how mutations occur (Figure 1).
Figure 1. Introduction to mutations and mutation testing*
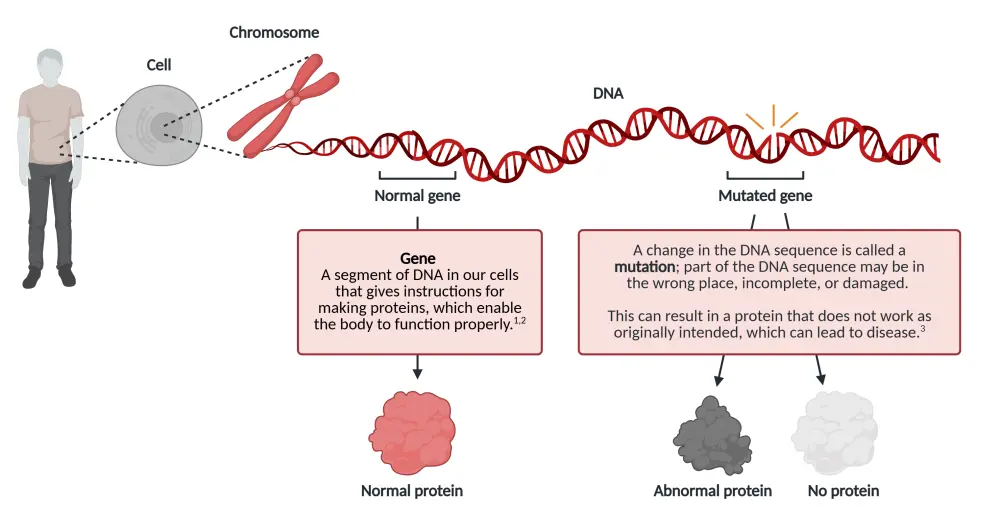
He then highlighted how mutation testing in AML helps to:
Confirm diagnosis, as certain mutations such as CEBPA, FLT3, IDH1/2, and NPM1 are strongly associated with acute leukemia.4
Categorize mutations and identify AML subtypes, which can help estimate how a patient might respond to treatment and predict the likely course of the disease.5
Guide the use of targeted therapies designed to target a particular mutation, such as FLT3.5
Align with clinical guidelines, as the current recommendations for diagnosis and management of AML now state that the characterization of mutations that commonly occur in AML with a technique called next-generation sequencing is essential.6,7
Make decisions around whether a stem cell transplant would be suitable.5
Detect any low levels of disease that may remain after treatment.8
This independent educational activity is supported by Thermo Fisher Scientific.
All content is developed independently by the faculty. The funder is allowed no influence on the content.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Charles Craddock
Charles Craddock Gail J. Roboz
Gail J. Roboz.webp&w=3840&q=75)
.webp&w=3840&q=75)
.webp&w=3840&q=75)

.webp&w=3840&q=75)
.webp&w=3840&q=75)