All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Liposomal drug delivery in acute myeloid leukemia
Do you know... Which liposomal drug formulation is approved for the treatment of patients with acute myeloid leukemia (AML)?
Acute myeloid leukemia (AML) is a heterogenous, aggressive myeloid malignancy, characterized by proliferation of blast cells and disruption of normal hematopoiesis.1,2 Despite considerable advances in understanding the pathobiology of AML, outcomes remain poor at the population level.2
Challenges with current treatment
Current clinical therapies for AML include chemotherapy, immunotherapy, targeted agents, and hematopoietic stem cell transplantation (HSCT).1 The “7+3 regimen”, a continuous 7-day infusion of cytarabine and a 3-day infusion of anthracycline (idarubicin or daunorubicin), has historically been the mainstay of induction treatment over the past 40 years;1,3 however, intensive chemotherapy regimens used in the induction and consolidation stages are not suitable for all patients, especially those with advanced age, secondary AML, serious underlying diseases, or poor physical fitness.1 Moreover, chemoresistance is becoming an increasing issue due to the heterogeneity and complexity of bone marrow.1 Therefore, therapeutic agents such as hypomethylating agents, B-cell lymphoma 2 (Bcl-2) inhibitor, isocitrate dehydrogenase 1 (IDH1) inhibitors, IDH2 inhibitors, and FMS-like tyrosine kinase 3 (FLT3) inhibitors have emerged as standard treatment approaches and are approved for the management of patients with AML. Despite these advancements, significant unmet needs remain, including drug toxicity, drug resistance, disease relapse, off-target adverse effects, and graft-versus-host disease.1
Liposomal drug delivery
To address current treatment gaps in AML, novel nanotechnologies—including liposomes, metallic nanoparticles, dendrimers, micelles, lipid nanoparticles, and polymeric nanoparticles—have emerged as promising approaches.3 Notably, liposomes are the first nanomedicine delivery system to progress from concept to clinical application and have been successfully adopted in clinical settings.1
Question 1 / 1
What components make up the bilayer arrangement of liposomes?
A
Proteins and carbohydrates
B
Carbohydrates and lipids
C
Nucleic acid and proteins
D
Phospholipids and cholesterol
Liposomes are spherical vesicles comprising phospholipids and cholesterol in a bilayer arrangement.3,4 Due to their amphiphilic nature, liposomes are capable of delivering both hydrophobic drugs (embedded within the phospholipid bilayer) and hydrophilic drugs (encased in the core).4 Figure 1 illustrates the basic structure of liposomes and their various types, and the potential benefits of liposomes are depicted in Figure 2.
Figure 1. Structure and types of liposomal drug delivery systems*
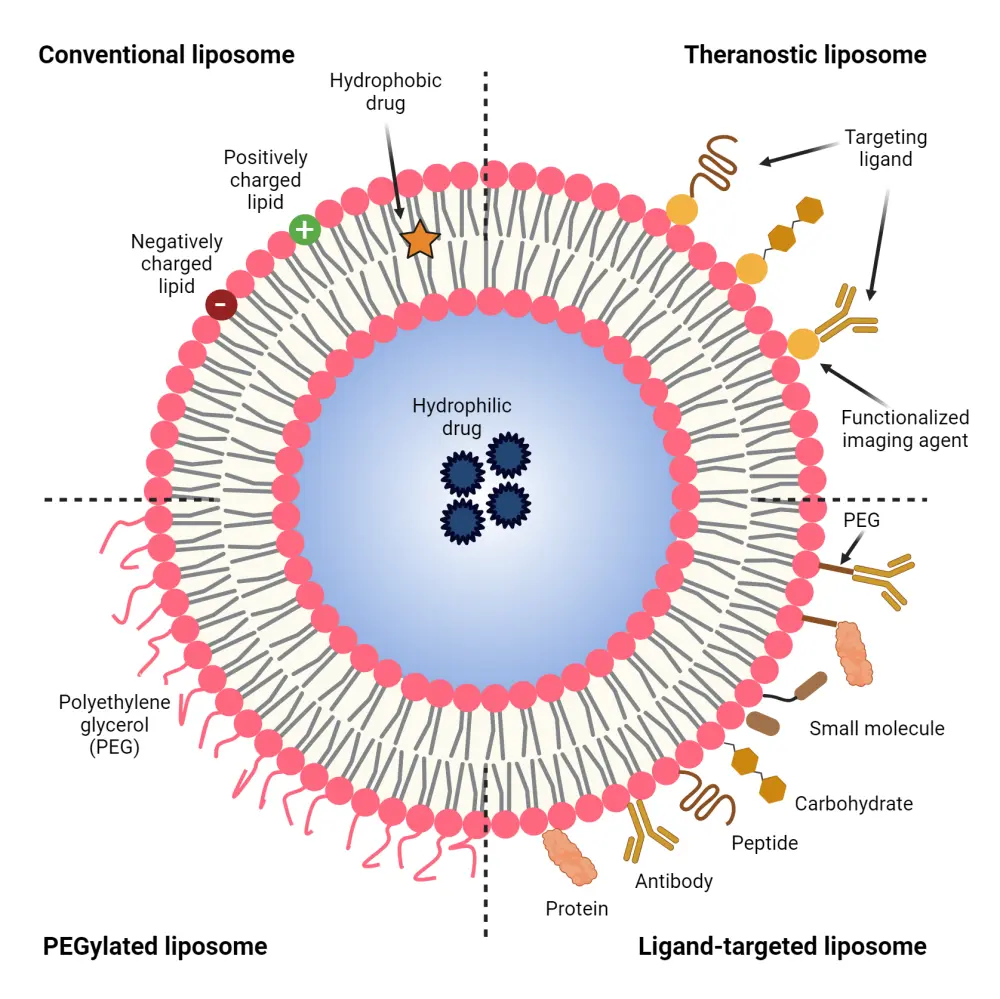
PEG, polyethylene glycol.
*Adapted from Benko, et al.4 Created with Biorender.com
Figure 2. Advantages of a liposomal drug delivery system*
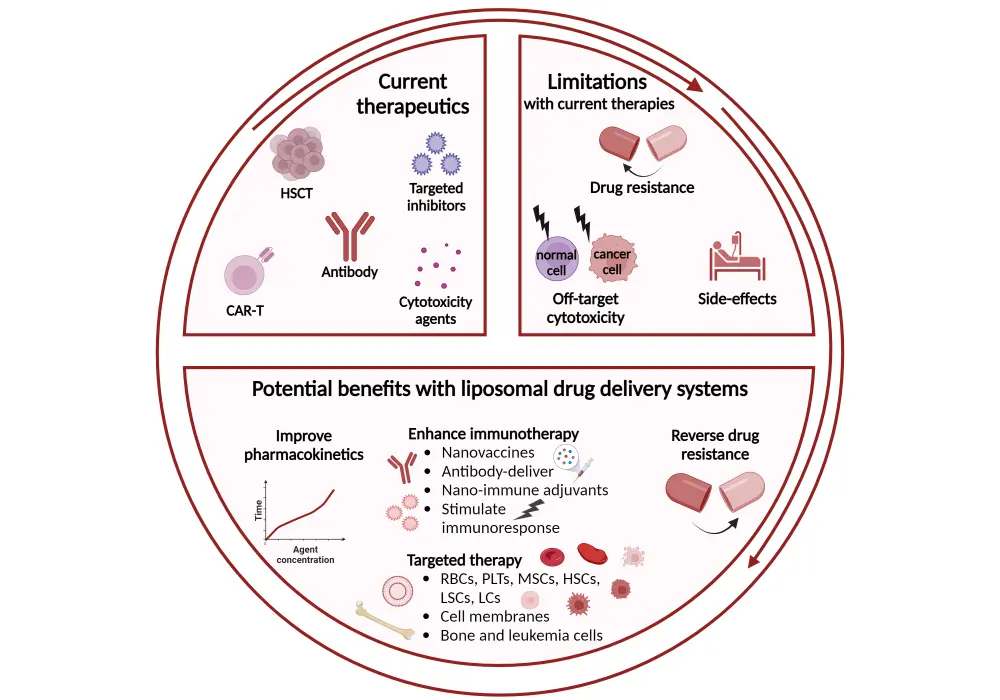
*Adapted from Wu, et al.1 Created with Biorender.com.
BM, bone marrow; CAR, chimeric antigen receptor; LC, leukemia cell; LSC, leukemia stem cell; MSC, mesenchymal stem cells; PLT, platelet; RBC, red blood cell.
Figure 3. Liposomal drug delivery mechanism*
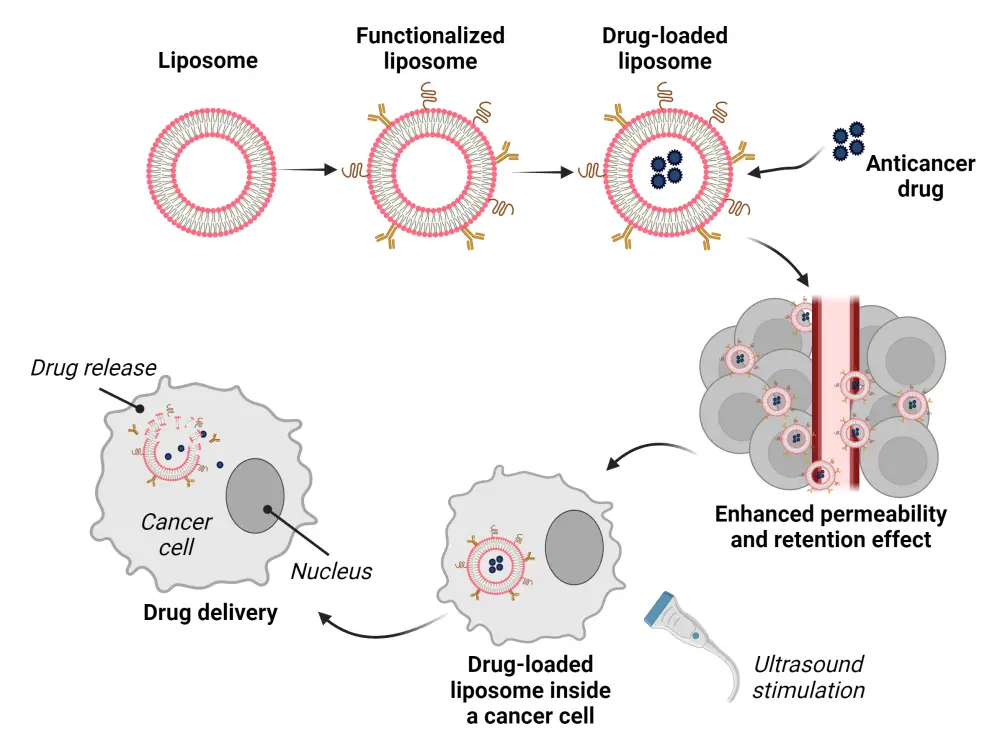
*Adapted from Benko, et al.4 Created with Biorender.com.
Figure 3 illustrates the mechanism of action of liposomal drug delivery systems. Liposomes can be functionalized using various methods, including antibodies, peptides, aptamers, proteins, small molecules, and hyaluronan, as shown in Figure 1, which can bind to specific receptors or antigens on leukemia cells and penetrate them. Liposomes can evade immune cell attacks by camouflaging plasma membranes from normal or leukemia cells, enhancing their uptake by leukemia cells through membrane fusion and increasing their targeting ability.1
Question 1 / 1
Which of the following best describes how liposomal formulations improve treatment efficacy in diseases, such as, AML?
A
By reducing the pH of the surrounding environment to activate the drug
B
By evading the immune response and increasing target cell permeability and retention
C
By enhancing the drug’s ability to bind directly to DNA within the target cells
D
By increasing the solubility of drugs in aqueous solutions
Approved liposomal drug formulations for AML
CPX-3515
The first liposomal products, such as those for daunorubicin or doxorubicin hydrochloride, were formulated with high cholesterol and encapsulated only a single agent.5 While these single-agent formulations had distinct pharmacokinetic profiles, the required fixed synergistic ratio could not be retained when administration was combined and manipulation of the liposomal composition was required to maintain a synergistic molar ratio for extended periods in vivo.5 To address this, CPX-351, a dual-drug low-cholesterol liposomal encapsulation of daunorubicin and cytarabine, was rationally designed.5
The structure of CPX-351 is shown in Figure 4. The liposome membrane is composed of distearoylphosphatidylcholine (DSPC), distearoylphosphatidylglycerol (DSPG), and cholesterol (CHOL) in a 7:2:1 molar ratio, giving the liposome a high melting point. It allows the liposomes to remain in a gel phase at body temperature and maintain the synergistic drug ratio in vivo for >24 hours post intravenous administration.5 The low level of cholesterol in the membrane helps in retaining hydrophilic substances and stabilizing the lipid bilayer. Cytarabine and daunorubicin are co-encapsulated in the liposome’s aqueous layer at a molar ratio of 5:1.5 Cytarabine is passively encapsulated into the preformed liposomes using copper gluconate buffer, while daunorubicin is subsequently actively encapsulated through complexation with the intra-liposomal copper at a ratio of 1:1 to 1:2, which helps to retain both drugs effectively within the liposome.5
CPX-351 has demonstrated its efficacy and safety in clinical trials (Table 1). In 2017, the US Food and Drug Administration approved CPX-351 liposome injection for the treatment of adult patients with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes.6 In 2021, the U.S. Food and Drug Administration (FDA) approved extended indication of CPX-351 to include pediatric patients (aged ≥1 year) with AML.7
Figure 4. Structure of CPX-351*
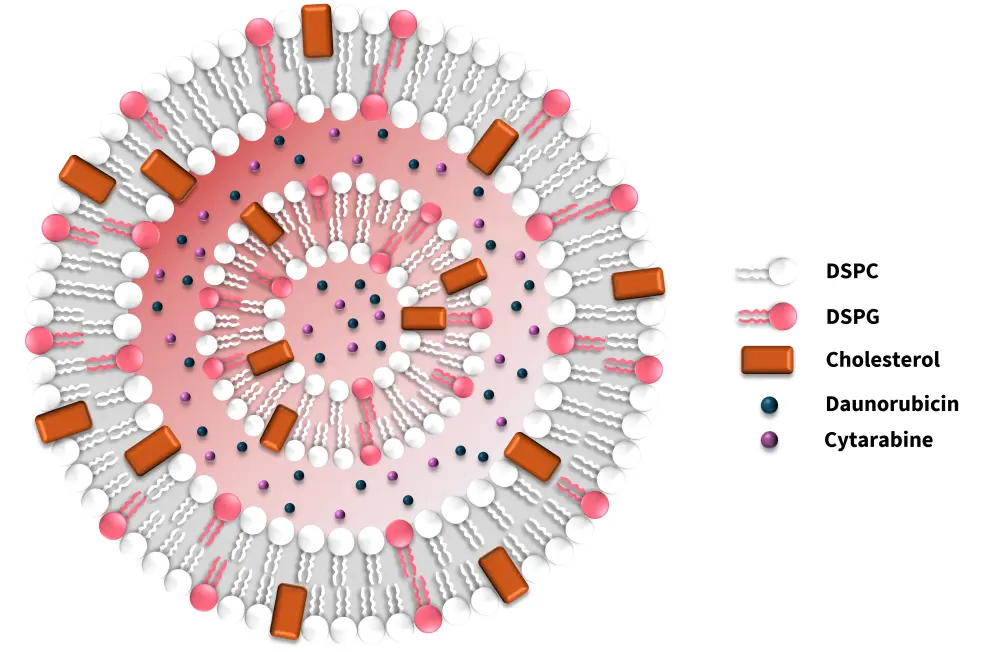
DSPC, distearoylphosphatidylcholine; DSPG, distearoylphosphatidylglycerol.
*Adapted from Mayer, et al.5
Table 1. Overview of clinical safety and efficacy of CPX-351 in AML*
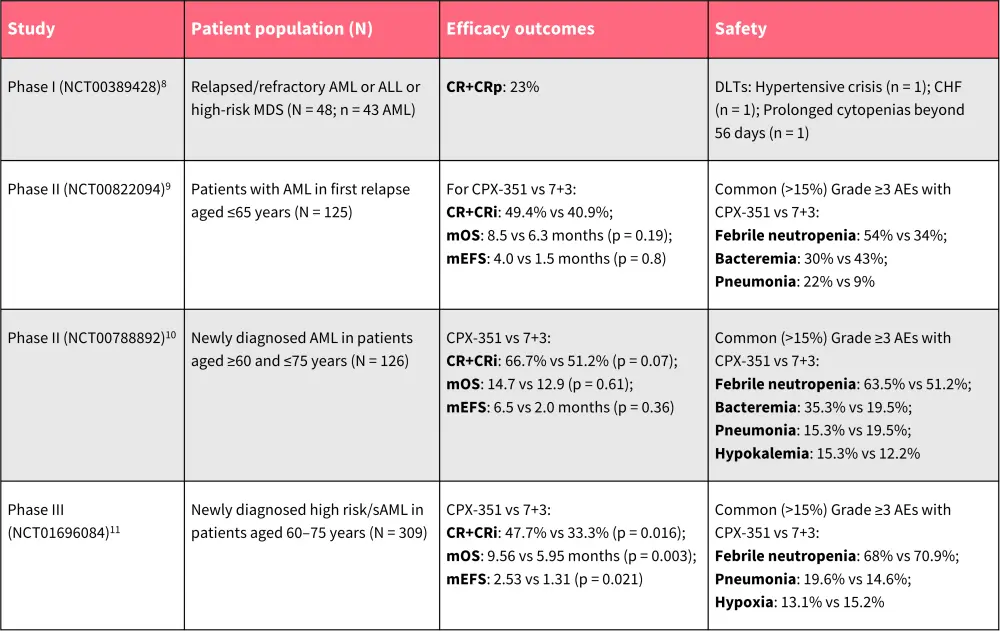
AML, acute myeloid leukemia; CHF, congestive heart failure; CR, complete remission; CRi, CR with incomplete neutrophil or platelet count recovery; DLT, dose-limiting toxicity; MDS, myelodysplastic syndromes; mEFS, median event-free survival; mOS, median overall survival; sAML, secondary AML.
*Data from Feldman, et al.8; Cortes, et al.9; Lancet, et al.10; and Lancet, et al.11
Learn more about patient health-related quality of life and real-world outcomes with CPX-351 here.
Question 1 / 1
CPX-351 (liposomal cytarabine and daunorubicin) offers several advantages over the conventional 7+3 cytarabine and daunorubicin treatment regimen for AML. How does the 5-year overall survival (OS) rate with CPX-351 compare to 7+3?
A
Half
B
About 10% higher
C
More than double
D
Approximately equal
To learn more about the advantages of CPX-351 over the conventional “7+3” treatment regimen, watch this expert opinion video: Does CPX-351 induce deeper responses compared to 7+3?
Liposomal formulations in development for AML
Prexigebersen (BP1001)
Growth factor receptor-bound protein-2 (GRB2) plays an essential role in cancer progression via the RAS activation pathway.13 Prexigebersen is a liposome-incorporated antisense oligodeoxynucleotide that targets GRB2.13 The liposomal formulation comprises of a P-ethoxy nucleic acid backbone and a neutral dioleoylphosphatidylcholine (DOPC) lipid delivery vehicle (Figure 5).14 The P-ethoxy backbone is formed by adding an ethyl group to the non-bridging oxygen atom of the phosphate bonds, producing a neutral, nuclease-resistant DNA antisense oligodeoxynucleotide that enhances its biodistribution and intracellular uptake.14
Figure 5. Structure of prexigebersen*
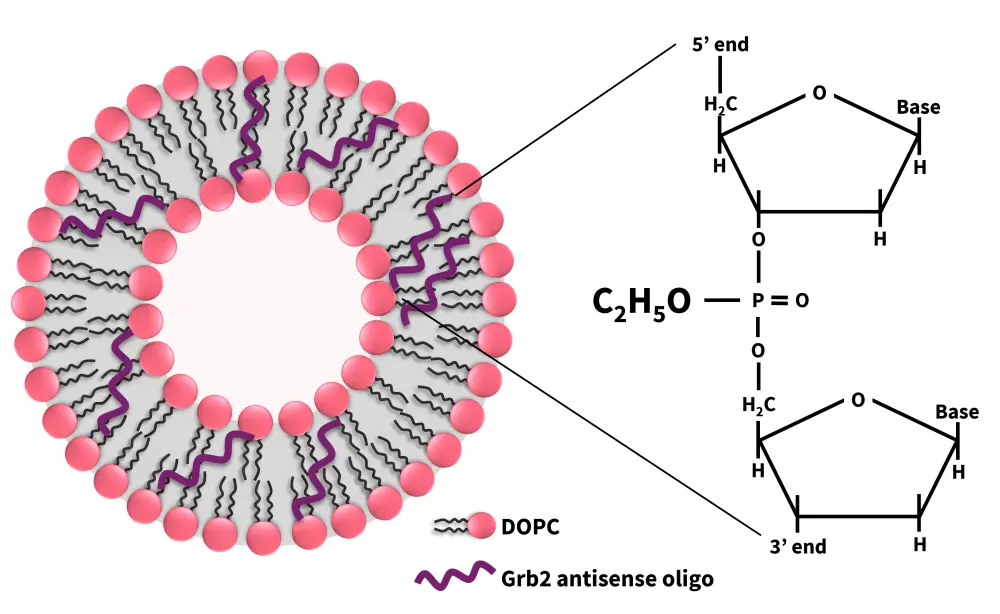
Grb2, growth factor receptor-bound protein-2; DOPC, dioleoylphosphatidylcholine.
*Adapted from Ohanian, et al.13
Table 2. Overview of clinical safety and efficacy of prexigebersen in AML*
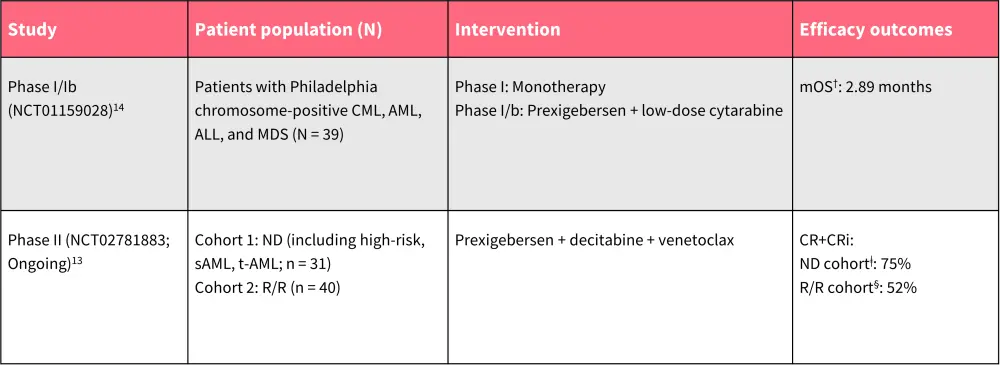
AE, adverse event; ALL, acute lymphoblastic lymphoma; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; CR, complete remission; CRi, CR with incomplete neutrophil or platelet count recovery; MDS, myelodysplastic syndromes; ND, newly diagnosed; R/R, relapsed/refractory; sAML, secondary AML; t-AML, therapy-related AML.
*Data from Ohanian, et al.13; and Ohanian, et al.14
†n = 27.
ǂn = 20.
§n = 23.
BP1002
BP1002 (liposomal Bcl-2 antisense oligodeoxynucleotide) is a neutral-charge antisense drug incorporated into liposomes, designed to inhibit the synthesis of Bcl-2, a protein that blocks programmed cell death.16,17 The FDA-approved Bcl-2 inhibitor venetoclax is used in frontline combination therapies for patients with AML ineligible for intensive chemotherapy; however, instances of resistance have been reported.16,17 A recent study revealed that patients with AML who relapsed following frontline venetoclax-based therapy were refractory to salvage therapy and had a very poor prognosis, with a median survival of less than 3 months.16,17 BP1002 could therefore be a potential treatment option for venetoclax-relapsed AML, as preclinical studies indicate that it works effectively with decitabine in venetoclax-resistant cells.16 A Phase 1/1b study (NCT05190471) is currently underway to evaluate BP1002 in combination with decitabine in patients with AML who have relapsed after venetoclax-based treatment.16,17
L-annamycin
Although anthracycline-based chemotherapy (e.g., “7+3”) is still a common induction approach in AML treatment, their use can be constrained due to inherent cardiotoxicity and drug resistance.18,19 L-annamycin (L-ANN) is a novel, doxorubicin congener encapsulated in multilamellar liposomes, designed to overcome multidrug resistance and avoid cardiotoxic effects.18,19 It is currently being investigated in a phase I/II MB-106 study (Table 3).
L-ANN has been granted Orphan Drug Designation by the EMA.20 L-ANN was previously granted Fast Track Designation21 and Orphan Drug Designation22 by the FDA for the treatment of patients with relapsed/refractory acute myeloid leukemia.
Table 3. Overview of clinical safety and efficacy of L-ANN in AML*
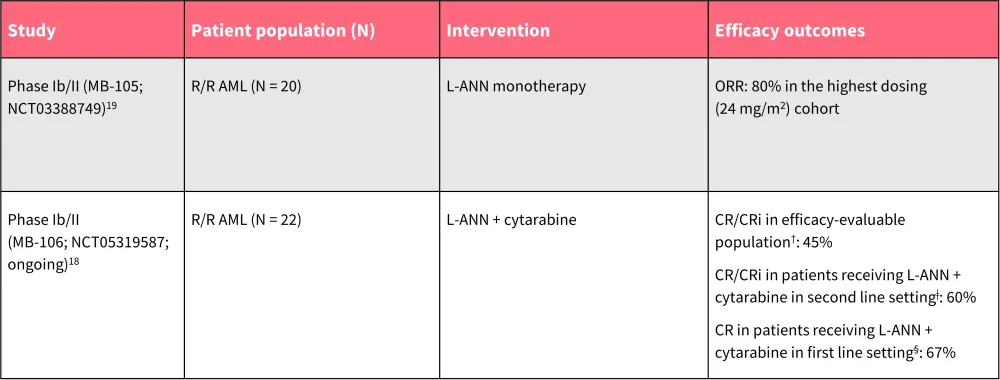
AE, adverse event; AML, acute myeloid leukemia; CR, complete remission; CRi, CR with incomplete neutrophil or platelet count recovery; L-ANN, L-annamycin; ORR, overall response rate; R/R, relapsed/refractory; TEAE, treatment-emergent AE.
*Data from Wadolowska, et al.18; and Gil, et al. 19
†n = 20.
ǂn = 10.
§n = 3.
Table 4. Ongoing trials evaluating liposomal drug delivery system in AML.
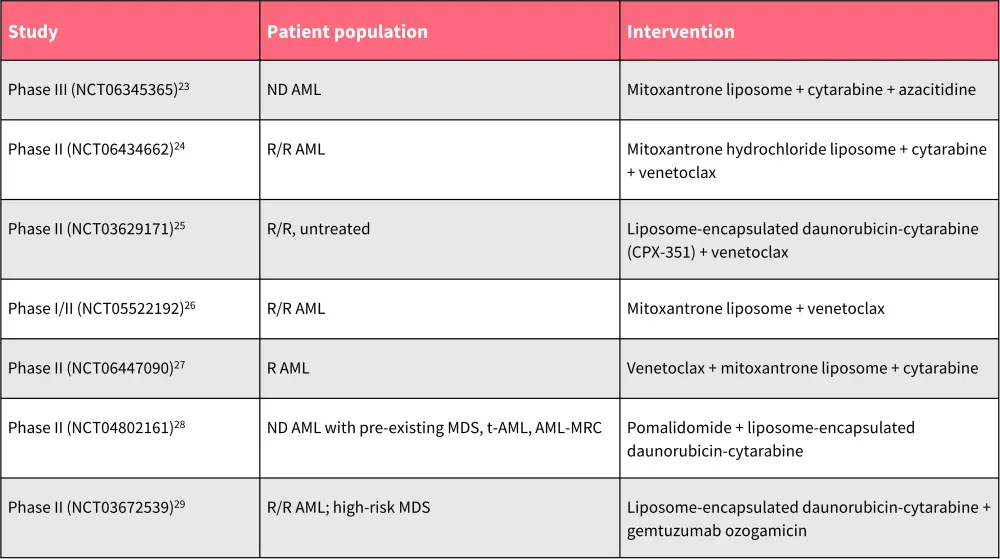
AML, acute myeloid leukemia; AML-MRC; AML with myelodysplasia-related changes; MDS, myelodysplastic syndromes; ND, newly diagnosed; R/R, relapsed/refractory; t-AML, therapy-related AML.
*Data from ClinicalTrials.gov.23–29
Other liposomal formulations in development
Table 4 provides a list of additional ongoing trials assessing liposomal drug delivery systems for AML.
Conclusion
The treatment landscape of AML is evolving, with the introduction of advanced liposomal drug delivery systems. By evading the immune response, and through enhanced cell permeability and retention, the novel liposomal formulations offer a promising approach to address the major treatment gaps in conventional therapies. Currently, CPX-351 is the only liposomal drug approved for the treatment of AML and is widely utilized in clinical practice, demonstrating improved efficacy compared with the standard “7 + 3” induction chemotherapy. Other liposomal formulations are in development, including prexigebersen and L-ANN, both of which have received Orphan Drug Designation from the FDA and EMA. Ongoing research in liposomal formulations marks a substantial step forward in management of AML, offering hope for more effective and personalized treatment options.
Your opinion matters
After reading the educational resource on liposomal drug delivery in AML, I commit to reviewing the latest data on CPX-351 and other liposomal drugs in development to guide my treatment of AML in clinical practice.
This educational resource is independently supported by Jazz Pharmaceuticals. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content






