All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
“How I Treat”: Case studies in relapsed/refractory acute myeloid leukemia
Do you know... Which of the following mutations should be used as a marker for measurable residual disease?
The “How I Treat” series, featured in Blood, highlights expert perspectives on the diagnosis and treatment of patients using sample patient cases, and the AML Hub has previously covered topics in these series on the use of new therapeutics and updated classification systems. Although there have been some major advancements in the treatment of patients with acute myeloid leukemia (AML) in recent years, relapsed/refractory (R/R) AML is still associated with poor outcomes.1
Here, we summarize key points from a recent “How I Treat” article by Thor et al.,1 discussing the treatment of patients with R/R AML, in three patient cases.
Treatment of R/R AML1
The 2022 European LeukemiaNet (ELN) recommendations include response criteria for R/R AML, incorporating both hematologic measures and the assessment of measurable residual disease (MRD). Molecular reevaluation should be carried out when diagnosing a patient with R/R AML as this may influence treatment decisions (Figure 1). Due to the poor outcomes associated with R/R AML, patients should be enrolled in clinical trials where possible (Figure 1). Allogeneic hematopoietic stem cell transplantation (allo-HSCT) rescues around one-third of R/R patients, and a donor search in all transplantable patients should be carried out if not already done at the initial diagnosis.
Figure 1. header...
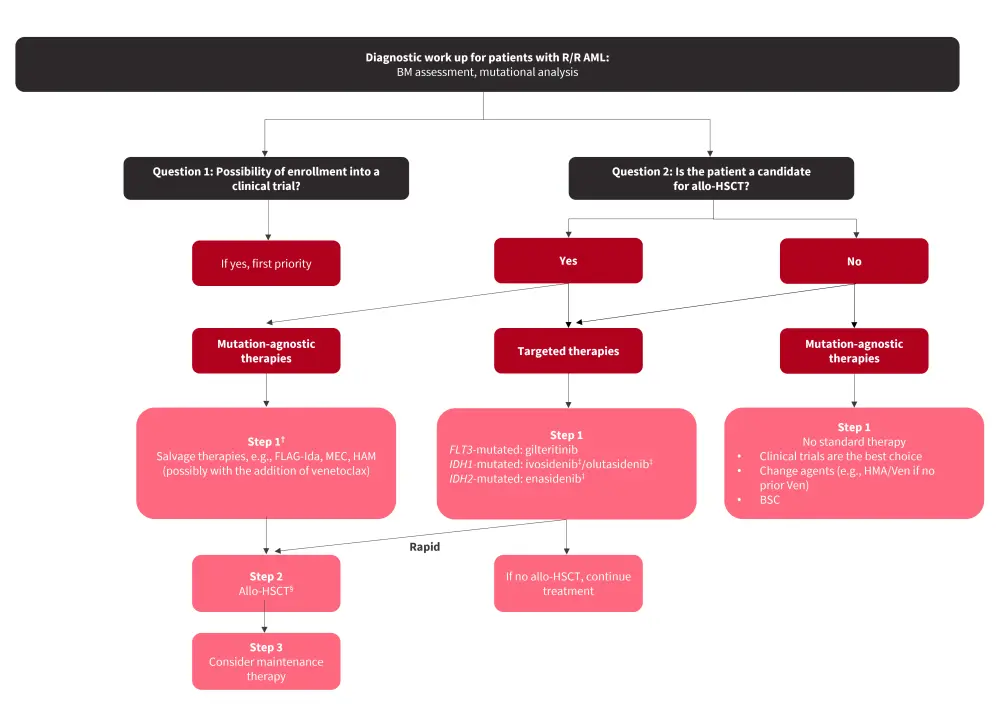
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; BM, bone marrow; BSC, best supportive care; FLAG-Ida, fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin; HAM, high-dose cytarabine, mitoxantrone; HMA, hypomethylating agent; MEC, mitoxantrone, etoposide, cytarabine; R/R, relapsed/refractory; Ven, venetoclax.
*Adapted from Thor, et al.1
†Some patients may go directly to allo-HSCT or receive lower-intensity regimens.
‡Not approved by the European Medicines Agency for patients with R/R AML.
Case 1. 52-year-old female
Figure 2. Case study 1 presentation*

AML, acute myeloid leukemia; FLT3, FMS‐like tyrosine kinase 3; ITD, internal tandem duplication; NPM1, nucleophosmin; TET2, tet methylcytosine dioxygenase 2.
*Adapted from Thor, et al.1
Created with BioRender.com.
Treatment
- Classified as favorable risk based on the 2017 ELN classification
- Received 7 + 3 plus midostaurin and achieved a complete remission (CR)
- Subsequently received 3 cycles of intermediate-dose cytarabine with midostaurin consolidation therapy
- NPM1 MRD was assessed using real-time quantitative polymerase chain reaction (RT-qPCR); MRD negativity was achieved
- 1-year post-consolidation, became MRD-positive with >400 mutated NPM1 copies/ABL × 104 in the bone marrow and >600 mutated NPM1 copies/ABL × 104 in the peripheral blood
Authors question
What does MRD relapse mean, and how can we clinically react to prevent morphological relapse?
- Based on the ELN recommendations, RT-qPCR is used for MRD assessment in patients with NPM1-mutated or core-binding factor AML, and multi-parameter flow cytometry is used for other AML subtypes
- While next-generation sequencing-based MRD assessment is rapidly evolving, clinical decisions should not be based on next-generation sequencing-based MRD assessment alone
- NPM1 is a robust MRD marker and can be detected at very low levels using RT-qPCR
- TET2 is a marker of clonal hematopoiesis and, similarly to the other DTA mutations (DNMT3A and ASXL1), should not be used as a marker of MRD
- FLT3-internal tandem duplication (ITD) is another clinically relevant MRD marker
- Once MRD positivity or an increase of MRD copy number ≥1log10 is detected by RT-qPCR in responding patients, results should be rapidly confirmed in a consecutive sample
- While there is no approved therapy or treatment standard for MRD relapse, donors should be identified for all transplant-eligible patients, and the authors recommend allo-HSCT in eligible patients with mutant transcript levels >200 NPM1 mutations/ABL × 104 and with confirmatory analysis, or enrollment in a clinical trial
Further treatment
- Morphological relapse occurred 4 weeks after MRD detection when genetic analysis confirmed an NPM1, FLT3-ITD (with an increase in the allelic ratio to 2.7), TET2, and a novel NRAS mutation
- Received gilteritinib monotherapy at 120 mg/day
- Achieved CR with incomplete count recovery and underwent allo-HSCT with a matched unrelated donor
- Posttransplant maintenance therapy included sorafenib; currently in CR 180 days posttransplant
Authors question
How do first-line therapy and mutational profile influence treatment options at relapse?
- Repeated molecular analysis is necessary to identify alterations of leukemic clones. In this case, the FLT3-ITD allelic ratio increased from 0.21 to 2.7; however, the FLT3-ITD clone is often lost at relapse following treatment with midostaurin, highlighting the need for molecular testing at relapse
- Although this patient was not initially a candidate for allo-HSCT due to their favorable risk, they received gilteritinib (an FLT3 inhibitor approved by the U.S Food and Drug Administration [FDA] and the European Medicines Agency [EMA] for the treatment of R/R FLT3-mutated AML) as a bridge to allo-HSCT
- In addition to another FLT3 inhibitor sorafenib, gilteritinib has also been investigated as maintenance therapy following allo-HSCT in the phase III MORPHO trial
- While the primary endpoint of relapse-free survival was not met, a benefit was observed in patients who were pre or posttransplant MRD-positive, suggesting that maintenance with a tyrosine kinase inhibitor could be tailored according to pre and posttransplant MRD status
- In fit patients without targetable mutations, salvage chemotherapy before allo-HSCT is recommended by the ELN and the National Comprehensive Cancer Network (NCCN) guidelines
- Novel agents such as the B-cell lymphoma 2 inhibitor venetoclax are being investigated in combination with salvage chemotherapy to improve responses, and the authors also highlighted the potential of fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin (FLAG-Ida) plus venetoclax
- Other future potential treatment options may include first-line venetoclax plus intensive chemotherapy, menin inhibitors such as revumenib and ziftomenib, and the E-selectin antagonist uproleselan
- Transplant-eligible patients typically receive salvage chemotherapy before transplant, although further studies are warranted to identify the molecular subgroups most likely to benefit from this approach
- The authors recommend that allo-HSCT is the highest priority for all transplantable patients with R/R AML
Case 2. 75-year-old male
Figure 3. Case study 2 presentation*

AML, acute myeloid leukemia; ASXL1, additional sex comb-like 1; IDH2, isocitrate dehydrogenase 2; NF1, neurofibromatosis 1.
*Adapted from Thor, et al.1
Created with BioRender.com.
Treatment
- Venetoclax and azacitidine combination therapy led to CR with incomplete count recovery following Cycle 1
- Received an additional 12 cycles of venetoclax and azacitidine, followed by a 10-week treatment holiday, requested by the patient
- On treatment reinitiation, 35% blasts were observed on blood smear
- Mutation analysis then revealed IDH2, ASXL1, NF1, and TP53 mutations
Authors question
What is the outlook for R/R AML patients after treatment with azacitidine/venetoclax, and what are their treatment options?
- Based on the VIALE-A trial, hypomethylating agents (HMAs) plus venetoclax is the standard of care for patients who are unfit for intensive chemotherapy, although this combination is not curative in most patients
- While patients with NPM1 mutations who are MRD-negative have favorable outcomes, most other patients become R/R with a poor prognosis
- If patients become transplant-eligible during treatment with HMAs plus venetoclax, allo-HSCT should be considered before relapse occurs
- For those who are R/R following HMAs plus venetoclax and ineligible for allo-HSCT, switching to other chemotherapy agents is not beneficial, and clinical trials should be considered alongside a discussion about the limitations of approved agents; best supportive care and palliative care should also be considered
- Targeted therapies, such as ivosidenib (IDH1), olutasidenib (IDH1), enasidenib (IDH2), gemtuzumab ozogamicin (CD33+), and gilteritinib (FLT3) are an option for patients with the relevant targetable mutation, although further studies are warranted to clarify their use in those who are R/R to HMAs plus venetoclax and there is also a need for novel treatments to improve the currently poor outcomes
Case 3. 65-year-old male
Figure 4. Case study 3 presentation*
AML, acute myeloid leukemia; DNMT3A, DNA methyltransferase 3 alpha; ECOG, Eastern Cooperative Oncology Group; NRAS, neuroblastoma RAS viral oncogene homolog; TP53, tumor protein p53.
*Adapted from Thor, et al.1
Created with BioRender.com.
Treatment
- Received one cycle of CPX-351, and achieved CR
- Underwent allo-HSCT from a matched unrelated donor, but relapsed 5 months later
Authors question
How could we prevent relapse after allo-HSCT in this patient, and what can we do if relapse occurs?
- The presence of a TP53 mutation and complex karyotype confers a high risk of relapse; allo-HSCT was recommended as the patient was fit and the risk of relapse exceeded 35–40%
- While allo-HSCT is the most effective consolidation therapy, relapse still occurs in 45–55% of patients with adverse risk AML, and the rate is even higher for patients with TP53 mutations
- The 2-year overall survival rate in patients who relapse after allo-HSCT is 14–25%, with particularly poor outcomes in those who relapse in the first 6 months
- While several ongoing trials are investigating the use of different maintenance therapies, there is currently no standard; the authors recommend reducing immunosuppressive therapy when possible and integrating donor-lymphocyte infusions
- Donor-lymphocyte infusions can be combined with other therapies, but the potential development of graft-versus-host disease must be assessed continually
- For a small number of patients, a second allo-HSCT may be possible
Conclusion
The landscape of treatment for patients with R/R AML is evolving. Triplet combinations of azacitidine and venetoclax plus targeted therapy or novel agents are currently being investigated, with promising initial results. Novel immunotherapies, including chimeric antigen receptor T cells, bispecific T-cell engaging antibodies, or dual-affinity retargeting antibodies are currently under evaluation. However, substantial effort is required to improve outcomes for this patient population.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

