All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Editorial theme | Navigating the latest updates in classification systems for AML: The 2022 ELN recommendations
Do you know... Which group of patients does the 2022 European LeukemiaNet (ELN) genetic risk stratification apply to?
The AML Hub has previously covered the 2022 European LeukemiaNet (ELN) recommendations for the diagnosis and management of acute myeloid leukemia (AML). These recommendations include updates to the classification of AML, an increased emphasis on genomic diagnostics, assessment of measurable residual disease (MRD), and treatment recommendations.1
During the European Hematology Association (EHA) 2023 Hybrid Congress, Döhner2 and Ossenkoppele3 discussed the latest updates to the ELN 2022 recommendations. As part of our editorial theme on navigating the updates in classifications systems for AML, we are pleased to summarize the key updates to ELN 2022 recommendations.
Genetic risk stratification and the genetic landscape in older patients with AML2
The 2022 ELN stratifies patients as favorable, intermediate, or adverse risk group based on genetic factors (Table 1). However, this stratification should not be solely based on genetic screening at diagnosis and can change during treatment based on MRD analysis. It is also important to note that this risk stratification is based on data from intensively treated patients and is not applicable to patients receiving non-intensive treatment.
Table 1. 2022 ELN risk classification by genetics at initial diagnosis*
|
Risk category |
Genetic abnormality |
|---|---|
|
ELN, European LeukemiaNet; ITD, internal tandem duplication. |
|
|
Favorable |
t(8;21)(q22;q22.1)/RUNX1::RUNX1T1 |
|
inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/CBFB::MYH11 |
|
|
Mutated NPM1 without FLT3-ITD |
|
|
bZIP in-frame mutated CEBPA |
|
|
Intermediate |
Mutated NPM1 with FLT3-ITD |
|
Wild-type NPM1 with FLT3-ITD (without adverse risk genetic lesions) |
|
|
t(9;11)(p21.3;q23.3)/MLLT3::KMT2A |
|
|
Cytogenetic and/or molecular abnormalities not classified as favorable or adverse |
|
|
Adverse |
t(6;9)(p23;q34.1)/DEK::NUP214 |
|
t(v;11q23.3)/KMT2A-rearranged |
|
|
t(9;22)(q34.1;q11.2)/BCR::ABL1 |
|
|
t(8;16)(p11;p13)/KAT6A::CREBBP |
|
|
inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)/GATA2, MECOM(EVI1) |
|
|
t(3q26.2;v)/MECOM(EVI1)-rearranged |
|
|
−5 or del(5q); −7; −17/abn(17p) |
|
|
Complex karyotype, monosomal karyotype |
|
|
Mutated ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, and/or ZRSR2 |
|
|
Mutated TP53 |
|
FLT3-ITD
Patients with AML with mutated FLT3-internal tandem duplication (ITD) are categorized as intermediate-risk, irrespective of allelic ratio and NPM1 mutational status. This is mainly due to methodological issues with standardizing FLT3-ITD allelic ratio, the role of MRD for NPM1 mutations, and the impact of midostaurin-based therapy on FLT3-ITD mutated AML.
Since the publication of the 2022 ELN recommendations, the use of next-generation sequencing (NGS)-based analysis of FLT3-ITD MRD has led to the improved prediction of survival outcomes. For example, data from the QuANTUM-First trial demonstrated the prognostic impact of FLT3-ITD MRD and variant allele frequency in patients treated with quizartinib.
Myelodysplasia-related mutations
Patients with AML with myelodysplasia-related mutations are categorized as adverse risk. Retrospective studies have shown that the poor prognosis is associated with myelodysplasia-related mutations irrespective of the patients' age. There is a significant overlap between genetically defined AML and the revised 4th edition of the World Health Organization (WHO) classification category of AML with myelodysplasia-related changes (AML-MRC), raising the question of whether these patients may benefit from CPX-351 treatment. The NCRI AML19 trial showed that CPX-351 significantly improved survival outcomes versus fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin (FLAG-Ida) in patients with high-risk AML with myelodysplasia-related mutations.
Older, unfit patients
The 2022 ELN risk stratification is not applicable to older, unfit patients. Data from the ASTRAL-1 and the VIALE-A trials suggests that the 2022 ELN risk stratification does not provide clinically meaningful outcome stratification in these older, unfit populations.
- The ASTRAL-1 trial showed that the genomic landscape in older patients with AML ineligible for intensive chemotherapy was different compared with younger patients.
- The most common mutations in older patients with a median age of 77 years included ASXL1, TET2, SRSF2, DNMT3A, RUNX1 and TP53.
- An oncogenic tree model with each node representing a gene mutation and each branch describing the evolution of pathways of leukemogenesis yielded a stable and reproducible oncogenetic tree (Figure 1).
- Multivariable analysis revealed that DDX41 (hazard ratio [HR], 0.41; 95% confidence interval [CI], 0.24–0.69; p < 0.001) was a favorable prognostic factor while FLT3-ITD (HR, 1.70; 95% CI, 1.21–2.40; p = 0.002), SRSF2 (HR, 1.36; 95% CI, 1.06–1.76; p = 0.017), and TP53 (HR, 1.59; 95% CI, 1.24–2.04; p < 0.001) were unfavorable prognostic factors.
- Clinical factors such as age (HR, 1.02; 95% CI, 1.01–1.04; p = 0.009), sex (HR, 1.32; 95% CI, 1.09–1.60; p = 0.004), Eastern Cooperative Oncology Group performance status score (HR, 1.55; 95% CI, 1.28–1.88; p < 0.001), and higher white blood cell count (HR, 1.63; 95% CI, 1.34–1.97; p < 0.001) were significant prognostic factors in older patients treated with hypomethylating agents (HMAs).
- A model using a backward selection procedure confirmed DDX41 mutations as favorable and FLT3-ITD and TP53 mutations as unfavorable risk factors.
- The VIALE-A trial in patients receiving venetoclax + azacitidine identified higher-, intermediate-, and lower-benefit groups with a median overall survival (OS) of >24 months, 12 months, and <6 months, respectively.
- A prognostic risk signature was developed using the mutational status of 4 genes, FLT3-ITD, NRAS, KRAS, and TP53 in patients receiving venetoclax + azacitidine. These included:
- Higher benefit group: FLT3-ITD wild-type (wt), K/NRASwt, TP53wt.
- Intermediate benefit group: FLT3-ITD or K/NRAS mutated, and TP53wt.
- Lower benefit group: TP53 mutated.
Figure 1. Oncogenetic tree model using data from the ASTRAL-1 trial*
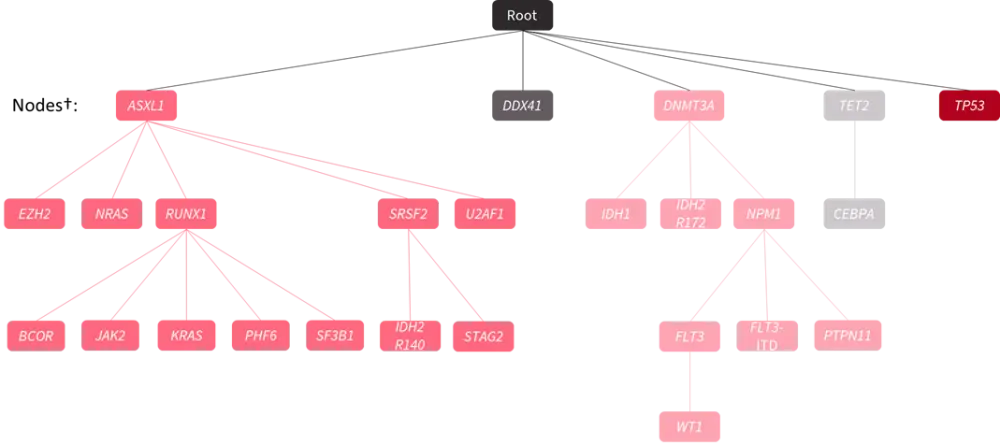
*Adapted from Döhner2 and Jahn et al.4
†Each node represents a gene mutation, and each branch describes the evolution of different possible pathways of leukemogenesis by inferring the sequence of mutation acquisition.
Genetic-driven treatment and novel clinical trial design3
The 2022 ELN recommendations have impacted the initial genetic workup in the diagnosis of AML. Gene mutations and rearrangements with clinical relevance should be screened at diagnosis as the presence of mutations influences the treatment decisions (Figure 2 and 3).
Figure 2. First-line treatment for fit patients in Europe based on genetic stratification*
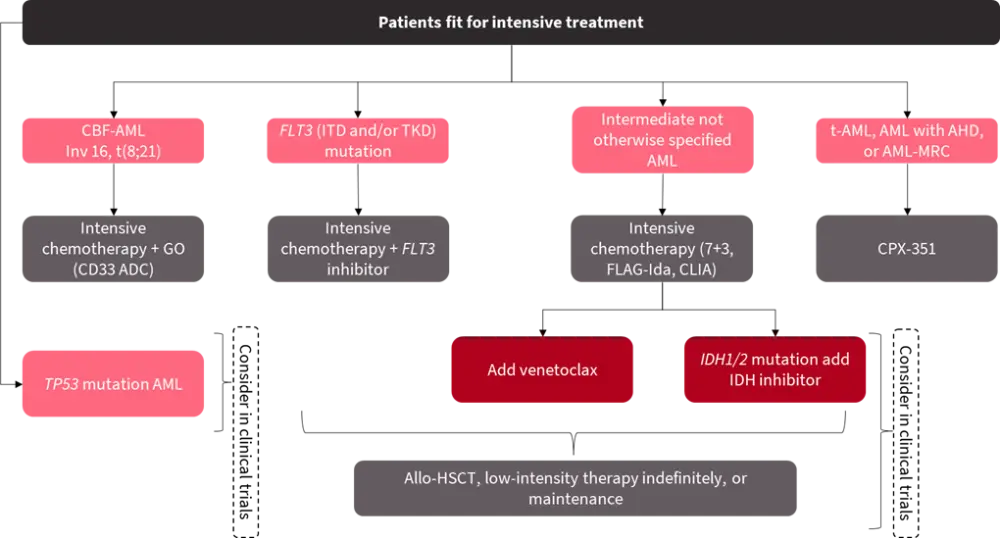
ADC, antibody drug conjugate; AHD, antecedent hematological disorder; allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; CBF, core binding factor; CLIA, cladribine, idarubicin, cytarabine; FLAG-Ida, fludarabine, cytarabine, granulocyte-colony stimulating factor and idarubicin; GO, gemtuzumab ozogamicin; ITD, internal tandem duplication; MRC, myelodysplasia-related changes; t-AML, therapy-related AML; TKD, tyrosine kinase domain.
*Adapted from Ossenkoppele3
Figure 3. First-line treatment for unfit patients in Europe based on genetic stratification*
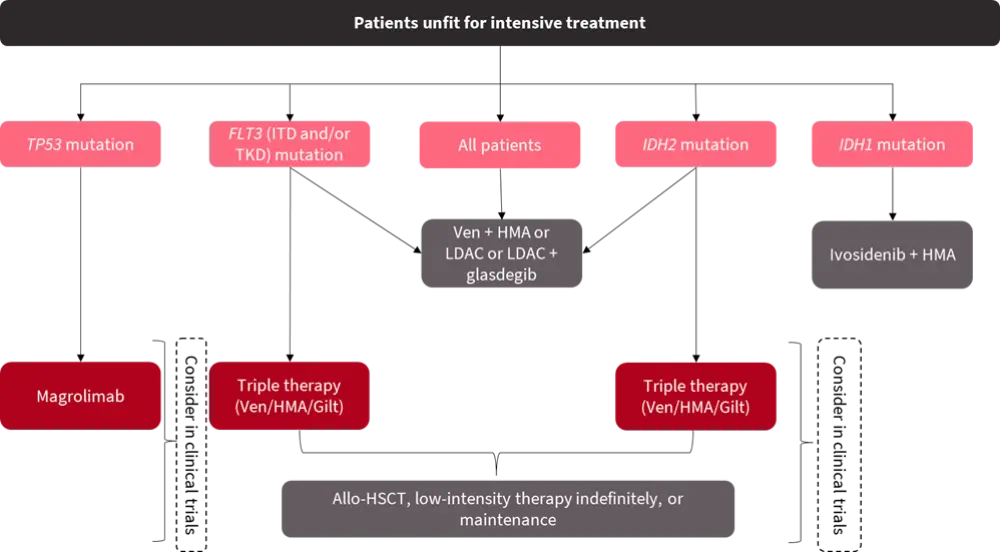
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; Gilt, gilteritinib; HMA, hypomethylating agents; ITD, internal tandem duplication; LDAC, low-dose cytarabine; TKD, tyrosine kinase domain; Ven, venetoclax.
*Adapted from Ossenkoppele3
Recent drug approvals
In the last 20 years, around ten new drugs have been approved for the treatment of AML (Table 2).
Table 2. Recent drug approvals for the treatment of AML*
|
AML, acute myeloid leukemia; EMA, European Medicines Agency; FDA, Food and Drug Administration; HMA, hypomethylating agent; ITD, internal tandem duplication; LDAC, low-dose cytarabine; MRC, myelodysplasia-related changes; R/R, relapsed/refractory; t-AML, therapy-related AML; TKD, tyrosine kinase domain. *Adapted from Ossenkoppele.3 |
|||
|
Drug |
Indication |
Approval |
|
|---|---|---|---|
|
FDA |
EMA |
||
|
Midostaurin |
First-line FLT3-ITD/TKD mutated AML |
Apr 2017 |
Sep 2017 |
|
Gilteritinib |
R/R FLT3-ITD/TKD mutated AML |
Nov 2018 |
Oct 2019 |
|
Quizartinib |
First-line FLT3-ITD mutated AML |
Jul 20235 |
Aug 2022 |
|
Gemtuzumab ozogamicin |
First-line (R/R) CD33+ AML |
Sep 2017 |
Apr 2018 |
|
CPX-351 |
t-AML/AML-MRC |
Aug 2017 |
Sep 2018 |
|
Glasdegib |
First-line frail AML + LDAC |
Nov 2018 |
Apr 2020 |
|
Venetoclax |
First-line frail AML + HMA/LDAC |
Nov 2018 |
May, 20216 |
|
Ivosidenib |
First-line R/R IDH1 mutated AML, first-line frail and IDH1 mutated AML + HMA |
Jul 2018, May 2019 |
May 2023 |
|
Enasidenib |
R/R IDH2 mutated AML |
Aug 2017 |
- |
|
Tagraxofusp |
Blastic plasmacytoid dendritic cell neoplasm |
Dec 2018 |
Nov 2020 |
Future efforts for innovative approaches in clinical trials
Collaboration
Between January 2000 and September 2020, 167 agents with 96 targets were investigated in 397 phase II trials for AML, suggesting the need for innovative approaches for investigating novel agents. Therefore, close collaboration between international trial groups is important to develop clinical trials with sufficiently large populations in a short time frame, such as in the HOVON 156/AMLSG 28–18 trial and the HOVON 150/AMLSG 29–18 trial. The EVOLVE consortium is a new group led by HOVON, AMLSG, and UK NCRI for studies of unfit patients with AML.
MRD assessment and statistical methods
Clinical trials need to be innovative in their approaches such as using surrogate endpoints (e.g., MRD) and statistical methods (e.g., Bayesian interim analysis). The prognostic impact of MRD in patients treated with intensive chemotherapy has been previously shown in a meta-analysis of 11,151 patients. MRD assessment can be useful to:
- Assess response.
- Refine prognostication and risk stratification.
- Inform treatment decisions.
- Identify patients at risk of relapse and guide early intervention.
- Serve as a surrogate endpoint in the future to enable the faster approval of novel agents.
The 2021 update on MRD in AML from the ELN MRD working group suggests that tailored treatment and/or conditioning regimen modification should be considered, particularly in clinical trials to reduce relapse in patients who are:
- MRD positive by multiparametric flow cytometry after 2 cycles of intensive chemotherapy, after completion of consolidation chemotherapy, prior to stem cell transplantation, after stem cell transplantation, and after stem cell transplantation.
- MRD positive by ≥2% or failed to achieve reduction of transcript levels by molecular analysis after completion of consolidation chemotherapy.
- Experiencing MRD relapse.
In the HO132 trial, MRD was used to guide treatment decisions, in patients with favorable or intermediate risk. Patients with favorable risk and MRD negative status received autologous peripheral blood stem cell transplantation, while patients with favorable or intermediate risk and MRD positive status received allogeneic peripheral blood stem cell transplantation.
Bayesian inference is a tool proposed for adaptive designs of clinical trials. It has the potential to strengthen the control arm leading to more rapid trial completion while allowing for benefit/risk assessment during trial accrual. The impact of Bayesian inference was assessed using the HO132 trial at four simulated interim analyses for event-free survival using matched patients from the preceding HOVON 102 trial to reinforce the control arm. The targeted HR of 0.76 for the primary endpoint of event-free survival was never reached at the four successive interim analyses versus observed HR of 0.99, suggesting that the probability of reaching a HR of 0.76 was very low.
Conclusion
Education around the correct implementation of the 2022 ELN recommendations is important for these guidelines to have the desired effect of improving clinical practice. Döhner2 highlights the need for comprehensive risk assessment incorporating both genetic screening at diagnosis and MRD analysis during treatment, the prognostic importance of NGS-based MRD assessment in patients with FLT3-ITD mutations, the relevance of AML with myelodysplasia-related gene mutations in treatment decisions, and that the 2022 ELN risk stratification is based on intensively treated patients and not applicable to older, unfit patients. He also discusses prospective studies identifying FLT3-ITD, NRAS, KRAS, TP53, and DDX41 as biomarkers for outcome stratification in patients treated with HMA-based therapies, and the potential of DDX41 to become a new marker for disease stratification.2
Ossenkoppele3 raises the need for comprehensive genetic testing with a short turnaround time, genetic-driven treatment approaches, and the need for international collaboration and novel innovative clinical trial design due to the updated AML classification.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content






