All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
MRD in AML: Prognosis, treatment, and surrogate endpoints
Your opinion matters
Do you see MRD negativity emerging as a surrogate endpoint across clinical trials in AML?
In recent years, the prognostic value of measurable residual disease (MRD) in patients with acute myeloid leukemia (AML) has been established, and early evidence suggests that MRD analysis may help to guide and personalize treatment decisions.1 At the 2021 Lymphoma, Leukemia & Myeloma Congress, Roland Walter, Fred Hutchinson Cancer Research Center, Seattle, US, gave a presentation on the current status of MRD analysis and how it can be employed in the AML setting.2 The AML Hub is happy to provide a summary.
Before you start, why not take a look at the video interview with Ali Bazarbachi at the American University of Beirut, Beirut, LB, on how MRD testing can be put to the best use in AML.
MRD in AML: Prognosis, treatment, and surrogate endpoints
Introduction2
Roland Walter opened with a summary of the currently available MRD identification methods (Table 1) before discussing promising emerging techniques such as circulating tumor DNA analysis, and highlighting the hurdles associated with currently employed techniques (Figure 1). Walter emphasized that different techniques generate complimentary, not identical, data, and that, until there is a greater understanding around the molecular drivers of relapse, this data remains incomplete. Despite disparities in MRD testing, it is an area of interest and expansion in AML, with aspirational uses in risk-stratification, personalized treatment decisions, and as surrogate endpoints in clinical trials. Therefore, research into MRD testing in AML is abundant.
Table 1. Conventional techniques for MRD analysis2
|
AML, acute myeloid leukemia; ddPCR, digital droplet PCR; qRT-PCR, quantitative reverse transcription-PCR; MFC, multiparameter flow cytometry; NGS, next-generation sequencing; PCR, polymerase chain reaction. |
|||||
|
|
MFC |
qRT-PCR /ddPCR |
NGS |
||
|---|---|---|---|---|---|
|
Detects |
Immunophenotypically abnormal cell populations. |
Single molecular abnormality. |
Multiple molecular abnormalities. |
||
|
Advantages |
- Applicable to >90% of cases; |
- Reproducible; |
- Applicable to >90% of cases; |
||
|
Disadvantages |
- Not all AMLs have abnormal immune phenotype; |
- Not widely applicable; |
- Requires error correction to overcome low sensitivity; |
||
Figure 1. Obstacles associated with conventional MRD analysis techniques*
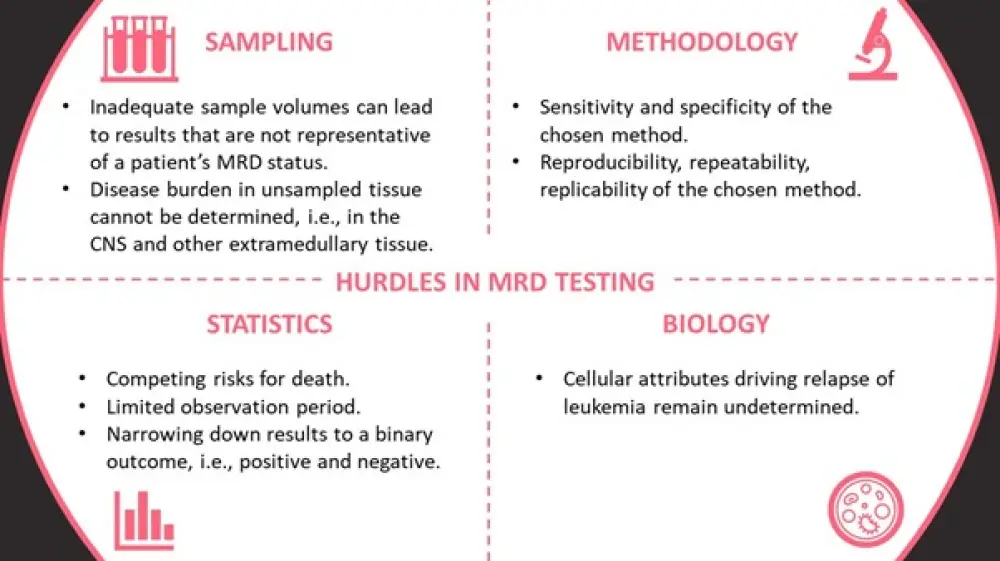
CNS, central nervous system; MRD, measurable residual disease.
*Information from Walter2
ELN MRD Working Party guidelines
Updated recommendations for measuring and inferring MRD in patients with AML were generated by the MRD Working Party of the European LeukemiaNet.3 An overview of the MRD assessment algorithm by AML phenotype is shown in Figure 2.
Figure 2. ELN MRD Working Party consensus guidelines for MRD assessment by AML phenotype*
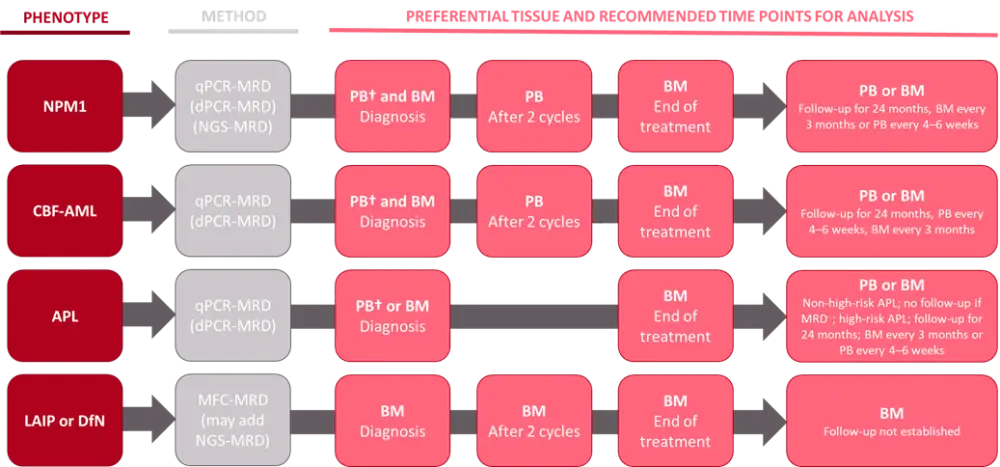
AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; BM, bone marrow; CBF-AML, core binding factor AML; DfN, different from normal; dPCR, digital polymerase chain reaction; LAIP, leukemia-associated immunophenotypes; MFC, multiparameter flow cytometry; NGS, next-generation sequencing; NPM1, nucleophosmin 1; PB, peripheral blood; PCR, polymerase chain reaction; qPCR; quantitative PCR.
*Data from Walter2 and Heuser3
MRD analysis and prognosis
Previously, morphologic assessment has been used to identify remission and predict the risk of relapse in patients with AML. MRD quantification offers a more robust and accurate prognostic tool and threatens to replace morphologic methods. MRD positivity has been strongly associated with poor outcomes to intensive chemotherapy, regardless of age, AML subtype, sample type, and MRD assessment method.3,4 Early data suggest that this remains the case for non-intensive chemotherapy regimens, including venetoclax plus azacytidine/decitabine.3
MRD-guided therapy
Currently, there is insufficient data supporting the use of MRD analysis to guide treatment decisions in the AML setting. However, there are a number of ongoing studies evaluating the feasibility of using MRD quantification to optimize patient outcomes to treatment. Areas that may lend themselves to MRD assessment include induction chemotherapy and conditioning regimen intensity, identifying the need for further pre-transplant chemotherapy or post-transplant therapy, and determining the type of post-remission therapy, optimal source of stem cells for transplantation, and choice of immunosuppressive agents.2
Roland Walter presented the findings from two studies to demonstrate how MRD may or may not be valuable in guiding treatment choices.2 Firstly, data from the BMT CTN 0901 study (NCT01339910) highlighted the benefit of myeloablative conditioning (MAC) over reduced intensity conditioning in patients with AML and myelodysplastic syndromes (MDS) receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT). Within the MAC cohort, the greatest benefit was observed in patients with MRD positivity at the time of transplant, supporting the use of MAC for MRD+ patients. Conversely, the FIGARO study (EudraCT: 2012-005538-12) did not identify differences in patient outcomes in patients considered MRD+ vs MRD−.2
MRD as a surrogate endpoint
Surrogate endpoints in clinical trials have been recognized by the U.S. Food and Drug Administration (FDA) and other regulatory bodies as grounds for both accelerated and traditional approvals, depending on its ability to predict clinical benefit.2
Arguments in favor of using MRD status as a surrogate endpoint include the meta-analysis of 11,151 patients with AML, which associated MRD negativity with significantly superior disease-free survival and overall survival, irrespective of age, AML subtype, sample type, time of MRD assessment, and MRD detection method.4 Additionally, the long-term assessment of MRD in patients enrolled in the QUAZAR AML-001 clinical trial (NCT01757535) associated post-induction MRD positivity with significantly shorter overall survival and relapse-free survival when compared with MRD− patients across both CC-486 and placebo arms.5
Conclusion
MRD quantification offers powerful prognostic information in patients with AML and techniques used for MRD analysis are continually being refined. Although the use of MRD as a surrogate endpoint and to guide treatment choices require validation, preliminary data suggest that there is a place for MRD testing in these fields.
To supplement this article, the AML Hub has put together a list of resources regarding the impact of MRD analysis on the management, treatment, and outcomes of patients with AML:
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

