All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
The latest developments on novel agents in the treatment of AML: Updates from #ASCO23 and EHA
Updates on several novel agents in acute myeloid leukemia (AML) were presented during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting and the European Hematology Association (EHA) 2023 Hybrid Congress. We are pleased to summarize updates on five novel agents being evaluated to treat patients with AML: iadademstat, ZN-d5, azenosertib, ziftomenib, and olutasidenib.
During ASCO 2023, Fathi1 presented an update of the FRIDA trial (NCT05546580) investigating iadademstat, previously covered by the AML Hub, plus gilteritinib. Smith2 presented an update of the ZN-d5-004C trial (NCT05682170) investigating ZN-d5 and azenosertib. During EHA 2023, Fathi3 presented the findings of the KOMET-001 trial (NCT04067336) studying ziftomenib monotherapy, previously reported by the AML Hub. Zeidan4 presented the findings of the KOMET-007 trial (NCT05735184) investigating ziftomenib combination therapy at ASCO 2023. During EHA 2023, Cortes5 presented a subgroup analysis from a phase II trial (NCT02719574) investigating olutasidenib, previously covered by the AML Hub. An overview of these trials is detailed in Table 1.
Table 1. Overview of studies presented at ASCO 2023 and EHA 2023*
|
AE, adverse event; AML, acute myeloid leukemia; BID, twice daily; CR, complete remission; CRh, CR with partial hematologic recovery; DLT, dose-limiting toxicity; KMT2Ar, KMT2A rearranged; ND, newly diagnosed; PO, per os (oral administration); QD, every day; RP2D, recommended phase II dose; R/R, relapsed/refractory. |
|||
|
Trial |
Intervention |
Design |
Primary endpoint(s) |
|---|---|---|---|
|
FRIDA |
Iadademstat with gilteritinib in adult patients with R/R FLT3-mutated AML |
Phase Ib trial. Iadademstat 100 µg/150 µg/75 µg PO 5 days, on 2 days off + gilteritinib 120 mg PO QD |
Safety, RP2D |
|
ZN-d5-004C |
Azenosertib monotherapy or in combination with ZN-d5 in patients with AML |
Phase I/II trial. Monotherapy: azenosertib up to 400 mg QD or alternative dosing schedule. Combination therapy: ZN-d5 (up to 1,000 mg QD) + azenosertib (up to 400 mg QD on a 5 days on, 2 days off schedule) |
AEs, DLTs |
|
KOMET-001 |
Ziftomenib in patients with R/R NPM1-mutated or KMT2Ar AML |
Phase I/II trial. Ziftomenib 600 mg QD |
CR/CRh |
|
KOMET-007 |
Ziftomenib in combination with venetoclax or venetoclax/azacitidine or standard cytarabine/daunorubicin 7+3 chemotherapy in patients with NPM1-mutated or KMT2Ar AML |
Phase I trial. Ziftomenib + venetoclax or venetoclax + azacitidine in R/R AML. Ziftomenib + cytarabine + daunorubicin (7+3) in ND AML |
Rate of DLT per dose level, AEs, CR |
|
2102-HEM-101 |
Olutasidenib in patients with R/R IDH1-mutated AML |
Phase II trial. Olutasidenib 150 mg BID alone or in combination with azacitidine |
CR/CRh |
Iadademstat1
Iadademstat is a specific, oral, potent, covalent inhibitor of the epigenetic LSD1/KDMA1 enzyme. The phase Ib ongoing FRIDA trial is investigating iadademstat + gilteritinib in adult patients with relapse/refractory (R/R) FLT3-mutated AML. In the dose-escalation portion of this study, up to ~6 patients per dose level will receive iadademstat in combination with gilteritinib. Once pharmacologically active doses are identified, these will be assessed in up to ~14 patients per dose cohort in the dose-expansion portion of this study and monitored for safety and efficacy (Figure 1)
Figure 1. FRIDA study overview*
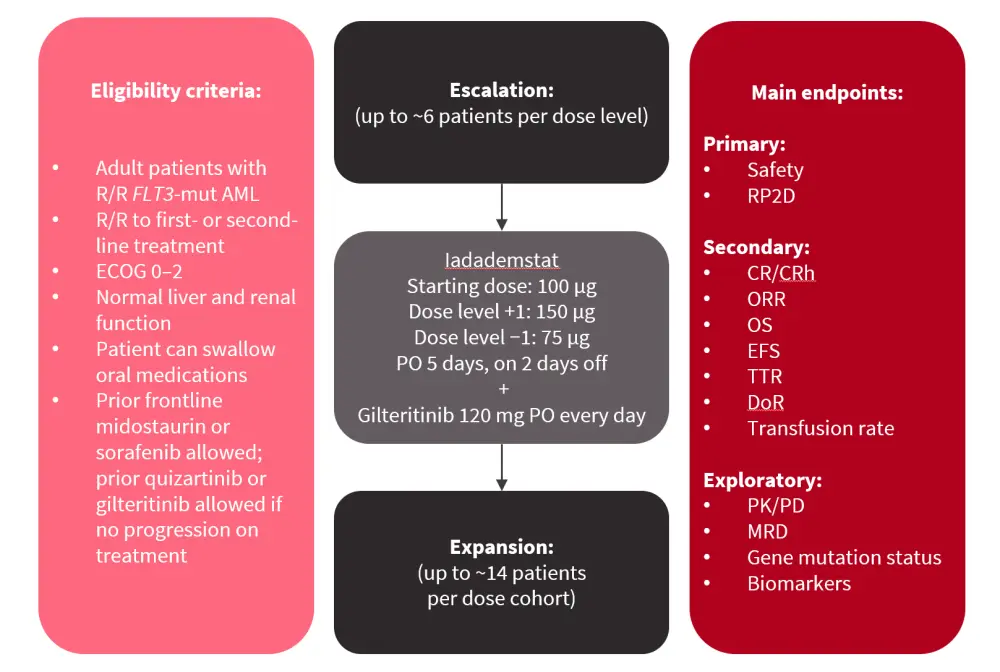
AML, acute myeloid leukemia; CR, complete remission; CRh, CR with partial hematologic recovery; DoR, duration of response; ECOG, Eastern Cooperative Oncology Group; EFS, event-free survival; mut, mutated; MRD, measurable residual disease; ORR, overall response rate; OS, overall survival; PD, pharmacodynamics; PK, pharmacokinetics; PO, per os (oral administration); RP2D, recommended phase II dose; R/R, relapsed/refractory; TTR, time to response.
*Adapted from Fathi.1
This study is planned to open in 15 sites in the U.S. and is currently accruing patients at four sites. The first dose-escalation cohort is completed, with no dose-limiting toxicities.
ZN-d5 and azenosertib2
ZN-d5 is an oral, selective BCL-2 inhibitor, while azenosertib is an oral, highly potent WEE1 inhibitor. The phase I/II ZN-d5-004C trial will assess ZN-d5 and azenosertib in patients aged ≥18 years with AML who are R/R to ≥1 line of therapy. Patients will receive azenosertib monotherapy or ZN-d5 + azenosertib combination therapy (Figure 2). The primary endpoints are adverse events (AEs) and observed dose-limiting toxicities. Secondary endpoints include:
- Rate and duration of remission
- Relapse rate
- Time to relapse
- Plasma pharmacokinetic parameters
Figure 2. Study overview*
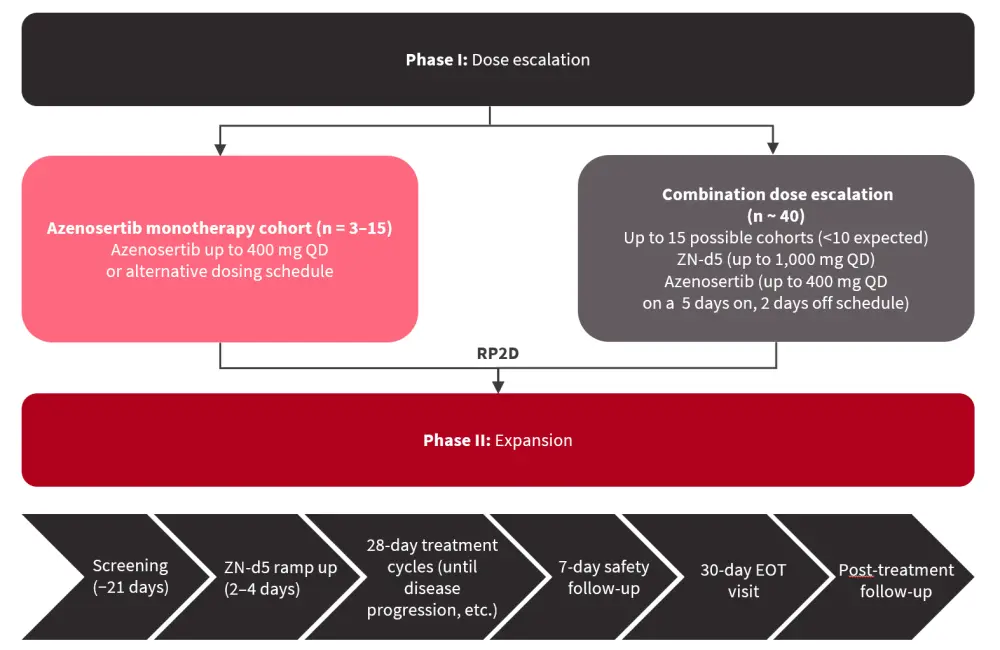
EOT, end of treatment; QD, every day; RP2D, recommended phase II dose.
*Adapted from Smith.2
This study is currently enrolling at seven centers in the U.S.
Ziftomenib3
KOMET-001
Ziftomenib is a potent and selective menin-MLL-KMT2A inhibitor.3,4 The phase I part of phase I/II KOMET-001 trial aimed to identify the recommended phase II dose of ziftomenib and assess the safety of ziftomenib in patients with R/R NPM1-mutated or KMT2A rearranged AML. The analysis presented by Fathi3 at EHA 2023 focused on the phase Ib expansion part of this trial (Figure 3).
Figure 3. KOMET-001 phase I/II overview*
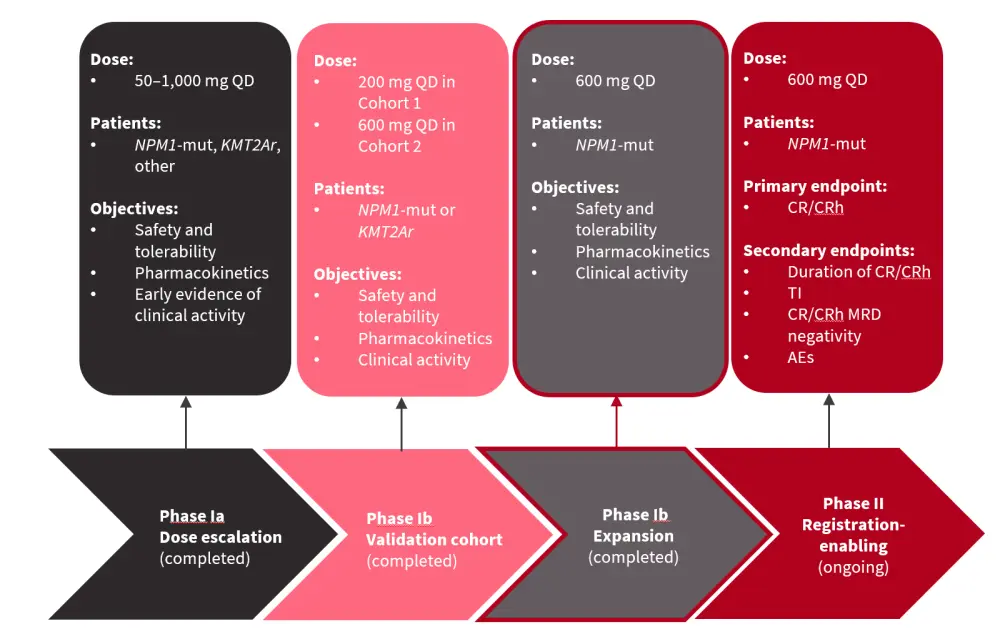
AE, adverse event; CR, complete remission; CRh, CR with partial hematologic recovery; KMT2Ar, KMT2A rearranged; MRD, measurable residual disease; NPM1-mut, mutated NPM1; QD, every day; TI, transfusion independence.
*Adapted from Fathi.3
The data cut-off for this analysis was April 12, 2023.
In total, 20 patients were included in phase Ib part of the trial, with a median age of 70.5 years. Of these, 30% of patients had a FLT3 co-mutation, 40% had an IDH1/2 co-mutation, and 20% had both FLT3 and IDH1/2 co-mutations. The median number of prior therapies was three, with 65% of patients previously treated with venetoclax and 20% of patients having had prior stem cell transplantation.
Key findings
Safety analysis revealed that 95% of patients experienced treatment-emergent AEs, including 85% of patients who experienced Grade ≥3 treatment-emergent AEs. In total, 60% of patients experienced treatment-related AEs, of which 30% experienced Grade ≥3 treatment-related AEs. The most common were nausea (20%) and differentiation syndrome (20%).
Ziftomenib was associated with a CR rate of 35%, and the presence of FLT3 and IDH1/2 mutations did not negatively impact response rates (Figure 4).
Figure 4. CR rate*
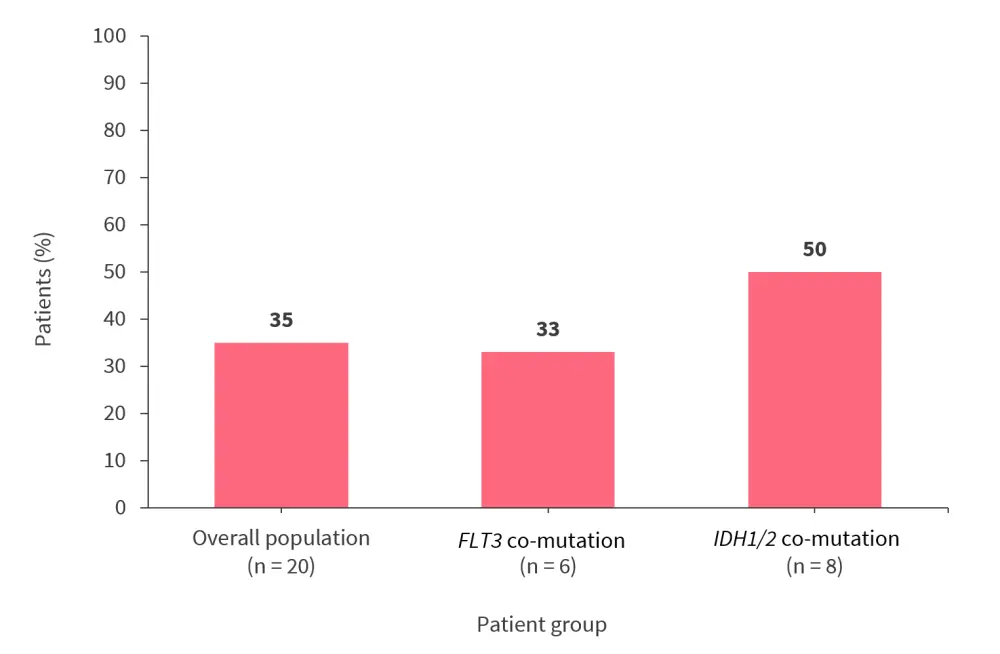
CR, complete remission.
*Data from Fathi .3
The composite CR rate was 40%, while the overall response rate (ORR) was 45%. The median time to first response was 51 days and the median duration of response was 8.2 months. Four of six patients achieving composite CR had negative measurable residual disease. The resistance profile was investigated, demonstrating that the binding affinity of ziftomenib is reduced by MeninM3271 but not by MeninT349M. The phase II part of KOMET-001 trial is currently recruiting patients with R/R NPM1-mutated AML.
KOMET-0074
The phase Ia/Ib KOMET-007 trial is a two-part dose-escalation and -expansion study investigating the safety, tolerability, and preliminary clinical activity of ziftomenib in combination with non-intensive and intensive chemotherapy in patients with NPM1-mutated or KMT2A-rearranged AML (Figure 5).
Figure 5. KOMET-007 study design*
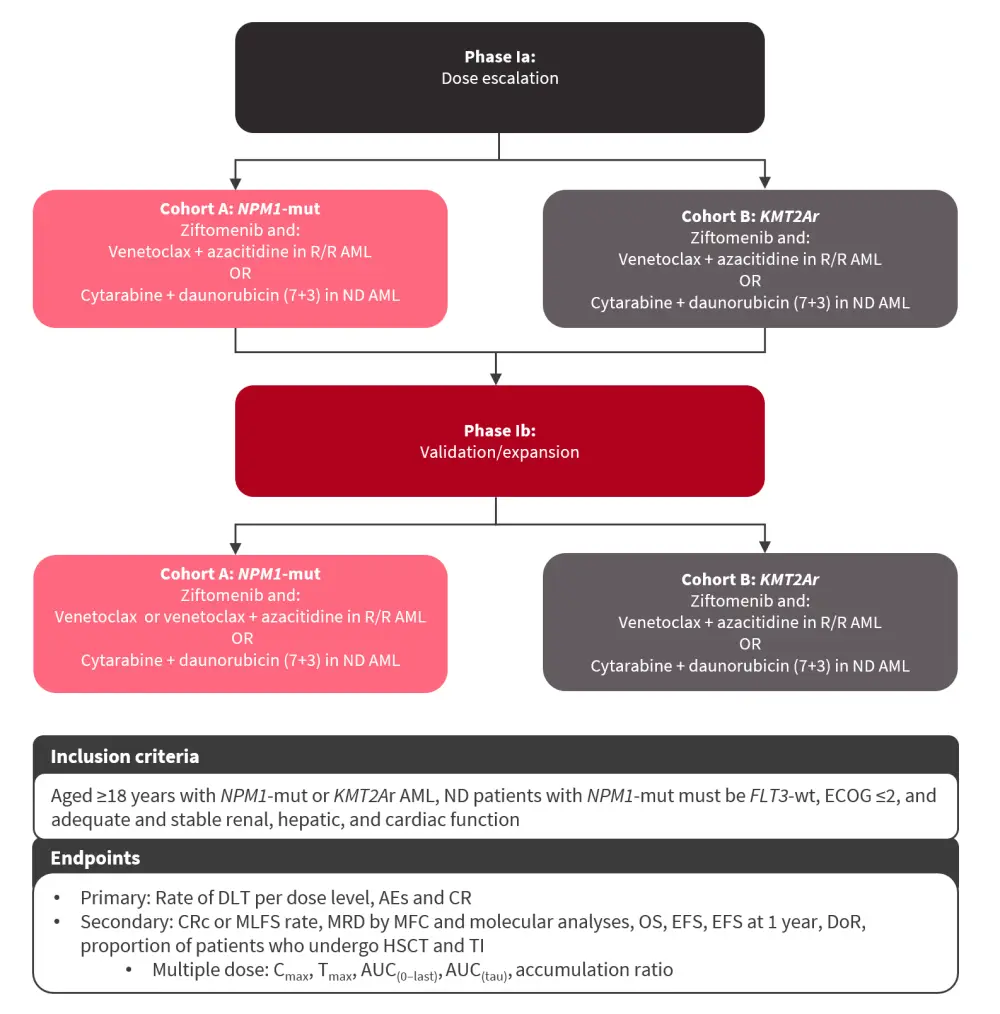
AE, adverse event; AML, acute myeloid leukemia; AUC(0–last), area under the concentration-time curve from time zero to the time of the last quantifiable concentration after dosing; AUC(tau), area under the concentration-time curve over a dosing interval; Cmax, maximum plasma concentration; CR, complete remission; CRc, composite CR; DLT, dose-limiting toxicity; DoR, duration of remission; ECOG, Eastern Cooperative Oncology Group; EFS, event-free survival; HSCT, hematopoietic stem cell transplantation; KMT2Ar, KMT2A rearranged; MFC, multiparameter flow cytometry; MLFS, morphologic leukemia-free state; MRD, measurable residual disease; ND, newly diagnosed; NPM1-mut, NPM1 mutated; OS, overall survival; R/R, relapsed/refractory; TI, transfusion independence; Tmax, time to maximum plasma concentration; wt, wild-type.
*Adapted from Zeidan.4
This study is currently enrolling at several sites across the U.S., with more sites to be added.
Olutasidenib5
Olutasidenib is an oral, potent, and selective IDH1 inhibitor. This is a subset analysis of 17 patients previously treated with venetoclax combinations from the 2102-HEM-101 trial. Patients received olutasidenib 150 mg twice daily either alone (n = 15) or in combination with azacitidine (n = 2) in a continuous 28-day cycle. The median age of patients in the post-venetoclax cohort was 73 years (range, 65–83 years) and the baseline characteristics are listed in Table 2.
Table 2. Patient characteristics*
|
HMA, hypomethylating agent. |
|
|
Characteristic, % (unless otherwise stated) |
Post-venetoclax cohort |
|---|---|
|
Study treatment |
|
|
Monotherapy |
88 |
|
Combination therapy |
12 |
|
Sex |
|
|
Male |
76 |
|
AML type |
|
|
Primary de novo |
41 |
|
Secondary |
59 |
|
Cytogenetics |
|
|
Favorable |
6 |
|
Intermediate |
53 |
|
Poor |
35 |
|
Unknown |
6 |
|
Prior therapy outcomes |
|
|
Refractory |
35 |
|
Relapsed |
53 |
|
Median number of prior treatments (range) |
2.0 (1–6) |
|
Prior HMA |
76 |
|
Prior venetoclax |
100 |
|
Induction |
88 |
|
Maintenance or salvage |
12 |
Key findings
The ORR was 41% in the entire post-venetoclax cohort, with an ORR of 40% and 50% in the monotherapy and the combination group, respectively. The CR/CR with partial hematologic recovery (CRh) rate was 29.4%, with 23.5% of patients achieving CR. Among patients who received prior venetoclax + azacitidine combination therapy, 50% achieved CR+CRh and 62.5% achieved an ORR. The median duration of CR+CRh has not been reached and is ongoing at 18.5 months (range, 4.8–not reached). Of the patients who were transfusion-dependent at baseline, 56-day transfusion independence was achieved by 25% and 43% of patients who were red blood cell and platelet transfusion-dependent, respectively.
Conclusion
These updates of ongoing trials presented at ASCO and EHA 2023 demonstrate promising novel therapies in the treatment of AML. The phase Ib expansion part of the KOMET-001 trial showed that ziftomenib was well tolerated and associated with significant clinical activity in patients with NPM1-mutated AML.3 In the subgroup analysis from the 2102-HEM-101 trial, olutasidenib was associated with durable remissions and transfusion independence in patients with R/R IDH1-mutated AML. Further findings from these trials will inform the efficacy and safety data on novel agents in the treatment of AML.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




