All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Inhibition of the menin-KMT2A interaction in AML: The AUGMENT-101 and KOMET-001 trials
Rearrangements in the KMT2A gene (KMT2Ar) are present in 5–10% of patients in the US diagnosed with acute myeloid leukemia (AML), and mutations in NPM1 (NPM1m) occur in ~30%.1 Moreover, ~10% of patients with acute lymphoblastic leukemia (ALL) and 80% diagnosed with infant ALL have KMT2Ar.2
The KMT2A protein, also known as MLL1, acts as a transcriptional regulator while NPM1 produces the multifunctional shuttling protein nucleophosmin.1 The interaction between KMT2Ar and the protein menin is instrumental in the expression of leukemogenic genes.1 This complex also interacts with the mutated NPM1 protein and causes abnormal expression of an overlapping set of genes involved in the development of leukemia, ultimately leading to oncogenic proliferation.1
There are currently no targeted treatments for patients with KMT2Ar, who frequently experience resistance to standard therapies; therefore, targeting the menin-KMT2A complex and subsequent downregulation of the genes HOXA9 and MEIS1 and reversal of leukemogenesis (Figure 1) is a promising approach.1
Figure 1. Inhibition of the menin-KMT2A complex*
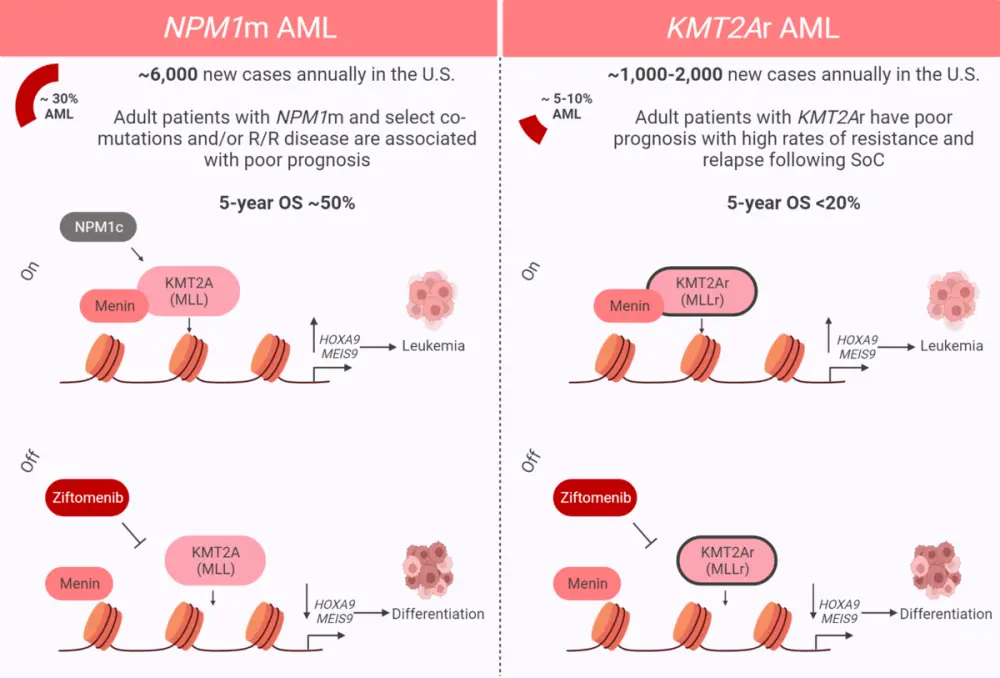
AML, acute myeloid leukemia; KMT2Ar, KMT2A rearrangement; MLLr, MLL rearrangement; NPM1m, NPM1 mutation; R/R, relapsed/refractory; SoC, standard of care.
*Adapted from Erba.1 Created with BioRender.com.
During the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, Issa2 presented findings from the phase I AUGMENT-101 trial investigating the menin-KMT2A inhibitor revumenib in patients diagnosed with relapsed/refractory (R/R) AML (NCT04065399), which has breakthrough therapy designation from the U.S. Food and Drug Administration (FDA). Preliminary results from this trial have been reported by the AML Hub. Issa3 also discussed outcomes after stem cell transplantation in patients from the same trial.
In addition, Erba1 presented results from the phase I/II KOMET-001 study investigating the menin-KMT2A inhibitor ziftomenib, also in patients with R/R AML (NCT04067336). We summarize the findings from these two trials below.
Revumenib in patients with R/R AML: Phase I results (AUGMENT-101)2
Study design
A total of 70 adult and pediatric patients with AML and KMT2Ar or NPM1m were included in this dose-escalation study with a rolling six design, whereby two to six patients were added to a dose level simultaneously. Revumenib was administered orally every 12 hours in 28-day cycles, and patients were stratified according to use of strong CYP3A4 inhibitors.
Results
The median age was 42.5 years; 82% of patients were diagnosed with AML, 16% with ALL, and 2% with mixed phenotype acute leukemia. A total of 68% of patients had KMT2Ar, 21% had NPM1m, and 12% had other genotypes.
All patients were heavily pretreated, with a median of four lines of treatment. Of the entire patient cohort, 46% had received prior transplantation before beginning treatment.
Response rates in the efficacy population (n = 60) with either KMT2Ar (n = 46) or NPM1m (n = 14) are shown in Figure 2, demonstrating the anti-leukemic activity of revumenib. Furthermore, 78% of patients had complete remission (CR)/CR with partial hematologic recovery and measurable residual disease (MRD) negativity.
Figure 2. Response rates to revumenib in the efficacy population (n = 60)*
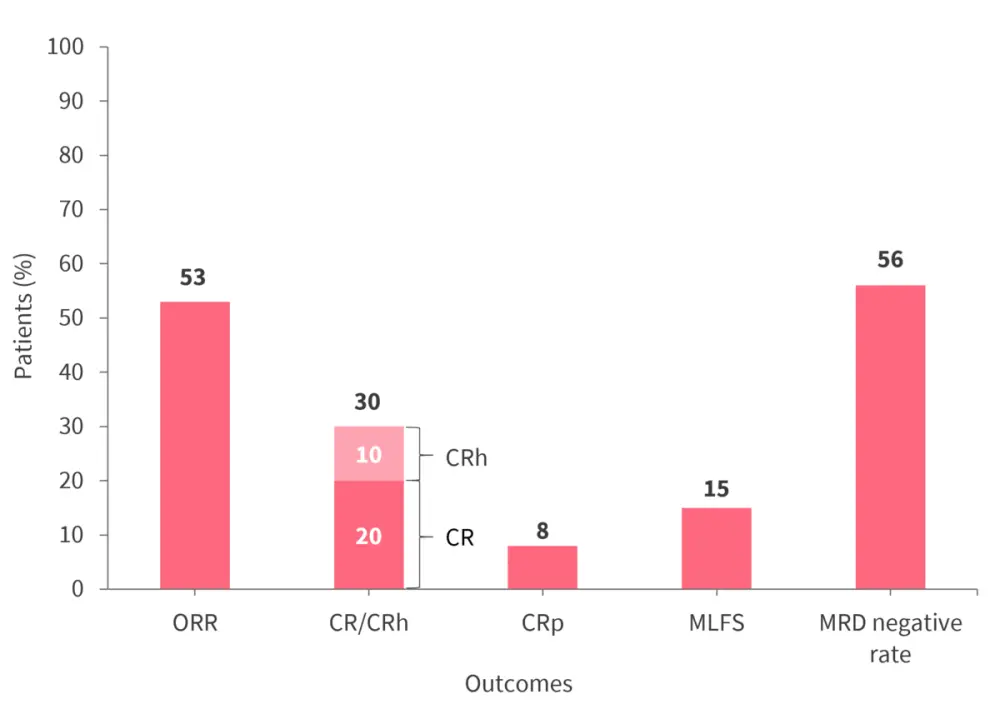
CR, complete remission; CRh, complete remission with partial hematologic recovery; CRp, complete remission with incomplete platelet recovery; MLFS, morphologic leukemia-free state; MRD, measurable residual disease; ORR, overall response rate.
*Data from Issa.2
Out of 32 patients who achieved an overall response rate, 18 (56%) were MRD negative. Furthermore, 59% of patients with KMT2Ar and 36% of patients with NPM1m achieved an overall response rate.
The median follow-up time was 4.6 months, median duration of response was 9.1 months (95% confidence interval, 2.7–not reached), and median time to response was 1.9 months. The median overall survival was 7 months (95% confidence interval, 4.3–11.6), with 12 patients proceeding to hematopoietic stem cell transplantation (HSCT). All patients with wild-type KMT2A and NPM1 did not show a response to revumenib.
Safety
Overall, 16% of patients in the safety population (patients who had received >1 dose of revumenib) reported Grade 3 treatment-related adverse events (TRAEs), the most frequently reported of which were:
- diarrhea (3%);
- anemia (3%);
- fatigue (2%); and
- tumor lysis syndrome (2%).
There were no TRAEs of any grade that led to treatment discontinuation or death. A total of 11 patients reported Grade 2 differentiation syndrome, but all were managed with steroid treatment with or without hydroxyurea. A total of 10% of patients had Grade 3 QTc prolongation seen on electrocardiogram at recommended phase II dosing levels.
Post-transplant outcomes3
A total of 12 patients (AML, 92%; ALL, 8%) proceeded to HSCT after achieving remission with revumenib. Of these patients, 83% had KMT2Ar and 17% had NPM1m. The median age was 35 years, all 12 patients were female, and 11 of these were MRD negative prior to HSCT. The outcomes following transplant for the whole subgroup were as follows:
- Remained in remission post-transplant, n = 9
- Remission for ≥ 1 year without additional maintenance therapy, n = 4
- Received revumenib maintenance therapy after HSCT, n = 3
- Relapsed, n = 2
- Death, n = 1
No new safety signals were identified, highlighting a favorable safety profile.
The AML Hub spoke with Issa about these findings here.
Ziftomenib in patients with R/R AML: Phase I/II KOMET-001 trial1
Study design
A total of 30 patients were included in the phase Ia dose-escalation part of the study and received 50–1,000 mg of ziftomenib. Furthermore, 53 patients were included in the phase Ib validation part of the study and received either 200 mg (n = 17) or 600 mg (n = 36) to determine the optimal phase II dose. The baseline patient characteristics for both parts of the study are shown in Table 1.
Table 1. Baseline characteristics*
|
HSCT, hematopoietic stem cell transplantation, KMT2Ar, KMT2A rearrangement; NPM1m, NPM1 mutation. |
|||
|
Characteristic, % (unless otherwise stated) |
Phase Ia |
Phase Ib |
Phase Ib |
|---|---|---|---|
|
Median age, years |
65.5 |
49 |
54.5 |
|
KMT2Ar |
33.3 |
76.5 |
44.4 |
|
NPM1m |
13.3 |
23.5 |
55.6 |
|
Median number of prior therapies, n |
3.5 |
3 |
3 |
|
Prior HSCT |
23.3 |
29.4 |
22.2 |
Efficacy
In phase Ia, one patient achieved CR, and a further patient achieved CR with MRD negativity. In addition, one patient reached morphologic leukemia-free state, and another with KMT2Ar reached a stable disease state with a significant decrease in blood counts lasting >4 months.
In phase Ib, 600 mg of ziftomenib showed anti-leukemic activity; response rates for patients treated with 200 mg or 600 mg of ziftomenib who had either NPM1m or KMT2Ar are shown in Figure 3. Out of the patients with NPM1m, one had MRD negativity at the 200 mg dose level and three had MRD negativity at the 600 mg dose level. In contrast, no patients with KMT2Ar had MRD negativity at the 200 mg dose level, while two had MRD negativity at the 600 mg dose level.
Figure 3. Response rates of patients treated with 200 mg or 600 mg ziftomenib with NPM1m or KMT2Ar*
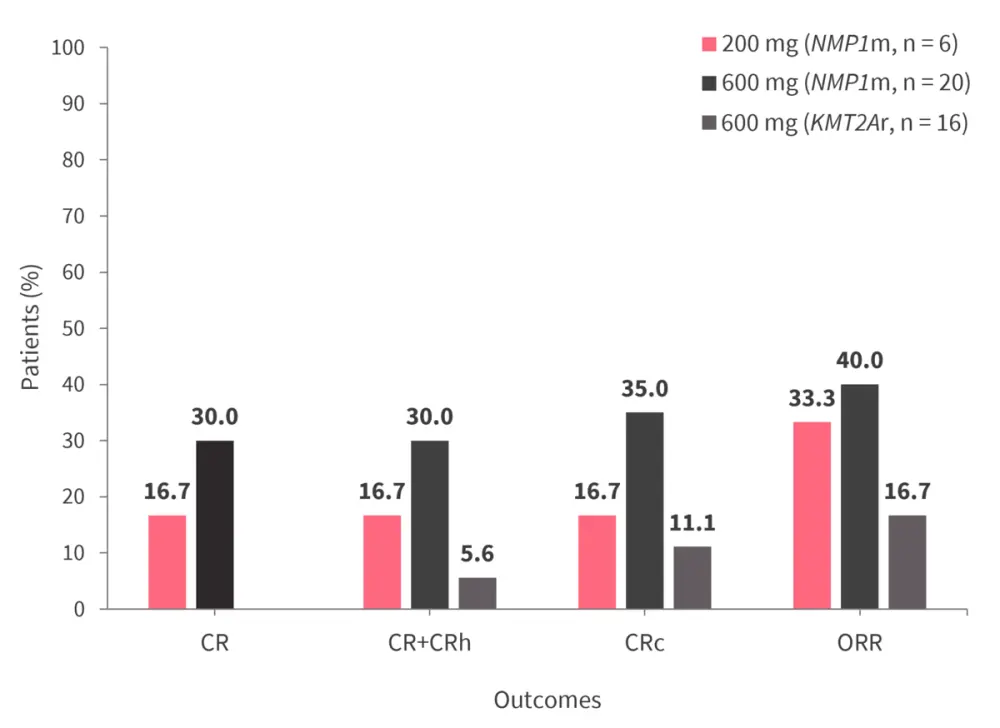
CR, complete remission; CRc, composite complete remission; CRh, complete remission with partial hematologic; KMT2Ar, KMT2A rearrangement; NPM1m, NPM1 mutation; ORR, overall response rate.
*Data from Erbas.1
Safety
In phase Ia, Grade 3 TRAEs were reported in ≥10% of patients and included the following:
- anemia (27%)
- pneumonia (23%)
- neutropenia (17%)
- thrombocytopenia (10%)
- decreased appetite (10%)
Two dose-limiting toxicities occurred; a case of pneumonitis at 400 mg ziftomenib and a case of differentiation syndrome at 1,000 mg ziftomenib that resulted in dose de-escalation. No drug-induced QTc prolongation was reported.
In phase Ib, at the 200 mg dose of ziftomenib, 30.8% of patients with KMT2Ar experienced ≥Grade 3 differentiation syndrome. At 600 mg, 25% of patients with KMT2Ar experienced ≥Grade 3 differentiation syndrome and 12.5% experienced ≥Grade 3 febrile neutropenia. No treatment-ending adverse events ≥Grade 3 were experienced by >10% of patients with NPM1m at either dose level.
Conclusion
Both revumenib and ziftomenib showed a manageable safety profile in the studies presented. Positive remission rates were observed with revumenib in heavily pretreated patients who were refractory to multiple lines of therapy, as well as in those who had received prior HSCT. Ziftomenib also had favorable preliminary efficacy in patients with R/R AML. In particular, the 600 mg dose of ziftomenib demonstrated efficacy in heavily pretreated patients and therefore has been recommended to be used in the phase II setting. These findings confirm that targeting the menin-KMT2A interaction has therapeutic potential in AML.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




