All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
CPX-351 as first-line treatment in AML: Real-world data
Do you know... Mutation of which the following genes has been shown to be a positive prognostic factor in patients with secondary AML receiving treatment with CPX-351?
CPX-351 as first-line treatment in AML: Real-world data
CPX-351, a liposomal formulation of cytarabine + daunorubicin, is approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of newly diagnosed patients with acute myeloid leukemia (AML) with myelodysplasia-related changes (AML-MRC) or therapy-related AML (t-AML). This approval was based on results from a phase III trial (NCT01696084) that demonstrated a survival benefit with CPX-351 vs conventional 7+3 chemotherapy in older patients with high-risk secondary AML (sAML).
Additionally, findings from the phase III UK AML19 trial (ISRCTN78449203) suggest a relapse-free survival benefit from CPX-351 vs fludarabine + cytarabine + granulocyte-colony stimulating factor + idarubicin (FLAG-Ida) in younger patients with high-risk AML or myelodysplastic syndromes (MDS), and an overall survival (OS) benefit in patients with AML with MDS-related mutations. However, real-world studies are needed to confirm the efficacy of CPX-351 when followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT),1 to determine the optimal duration of treatment with CPX-351 and timing of allo-HSCT,2 and to fully elucidate the prognostic impact of unfavorable gene mutations.3,4
In addition, the updated 5th edition of the World Health Organization (WHO) Classification of Haematolymphoid Tumours and the 2022 International Consensus Classification (ICC) of Myeloid Neoplasms and Acute Leukemias now include MDS-related mutations in the criteria for AML myelodysplasia-related (AML-MR, formerly AML-MRC).5 Further data are necessary to determine the efficacy of CPX-351 in younger patients with AML-MR and whether patients with mutation-defined AML-MR (AML-MRmut) have similar responses to other AML-MR subgroups.5 Below, we summarize several posters presented during the European Hematology Association (EHA) 2024 Hybrid Congress, by Avenoso1, Guolo2, Todisco, 3Riva4, and Peters,5 which discuss real-world outcomes of patients with AML treated with CPX-351.
Real-world outcomes of CPX-351 followed by allo-HSCT1
Study design and patient population
This analysis included patients with AML-MRC (n = 92) and t-AML (n = 18) who received upfront CPX-351 induction at five centers, between 2018 and 2023. The median age was 64 years, with 68% of patients aged ≥60 years. The primary endpoint was OS.
Key findings
- After a median follow-up of 10 months, the overall response rate (ORR) was 69%.
- Neutropenic fever occurred in 60% of patients, leading to 6 deaths.
- Median OS was higher in patients who received allo-HSCT (n = 52) vs no allo-HSCT (n = 52; p < 0.0001) and in patients aged ≤60 vs ≥60 years (p = 0.008; Figure 1).
- In patients who received allo-HSCT, median OS was not reached for either age group.
- Multivariable analysis confirmed that patients who did not receive allo-HSCT had a lower OS (p < 0.0001).
- In patients with a TP53 mutation (n = 17), median OS was 6 months, and 21 months vs 5 months in patients who received allo-HSCT vs did not receive allo-HSCT, respectively.
- Overall relapse rate was 20%, with a median time to relapse of 11 months.
Figure 1. OS in patients who received CPX-351 induction*
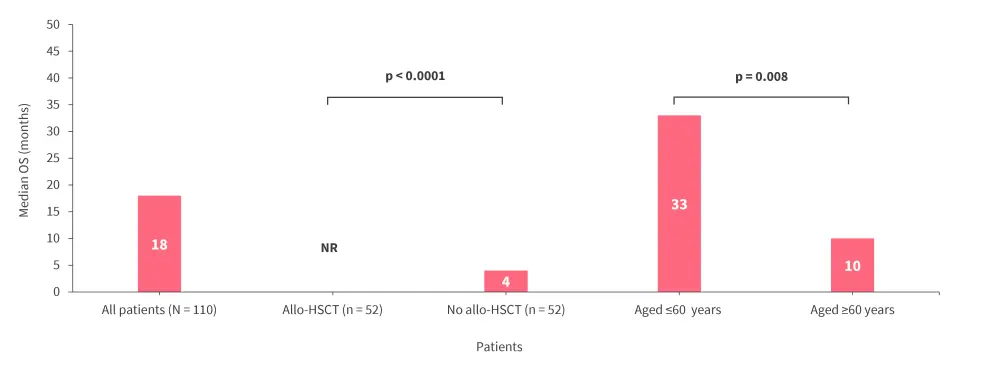
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; NR, not reached OS, overall survival.
*Data from Avenoso.1
These real-world data further confirm the efficacy of CPX-351 in patients with high-risk AML and highlights the survival benefit of allo-HSCT consolidation in patients with AML-MRC or t-AML.
Optimal duration of CPX-351 treatment, timing of allo-HSCT, and impact of TP53 mutations on response to CPX-3512,3
Study design and patient population
This study included 513 patients with sAML (t-AML, n = 108; AML-MRC, n = 405) who were treated with CPX-351 in 38 centers in Italy, from January 2019. The median age was 65.6 years, and 6% and 4.6% of patients had NPM1 and FLT3-internal tandem duplication (ITD) mutations, respectively. Of, 335 patients evaluated for the presence of TP53 mutations, 15% were TP53 mutation-positive, and 33% of these had a concomitant complex karyotype (CK). Patients were stratified by 2017 European LeukemiaNet (ELN) risk, with 5.2%, 34.5%, and 60.3% of patients having favorable, intermediate, and adverse risk, respectively.
Key findings: CPX-351 consolidation cycles and allo-HSCT2
- The complete remission (CR) rates after induction Cycles 1 and 2 were 58% and 66.3%, respectively, and 48.8% of patients received allo-HSCT in first CR.
- The CR rates were higher in patients with mutated NPM1 vs NPM1 wild type (wt) (p < 0.05), and also in favorable risk patients (p < 0.05).
- After a median follow-up of 23.66 months, the median OS was 16.23 months.
- The median OS was higher in patients with mutated NPM1 vs NPM1-wt (24.6 months; p < 0.05) and in patients with favorable risk (not reached; p < 0.05).
- In the landmark analysis of patients alive and in CR at Day 90, allo-HSCT was the strongest predictor of OS (median OS not reached vs 16.3 months for allo-HSCT vs no allo-HSCT, respectively; p < 0.05).
- Receiving 2 cycles of CPX-351 consolidation was beneficial vs <2 cycles in patients not proceeding to allo-HSCT (p < 0.05) but not in patients who received allo-HSCT (Table 1).
Table 1. Impact of CPX-351 consolidation cycles on median OS in patients who received and did not receive allo-HSCT*
|
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; OS, overall survival. |
||
|
Median, months |
OS |
p value |
|---|---|---|
|
No allo-HSCT |
|
|
|
2 cycles of CPX-351 consolidation |
20.36 |
< 0.05 |
|
<2 cycles of CPX-351 consolidation |
12.2 |
|
|
Allo-HSCT |
|
|
|
2 cycles of CPX-351 consolidation |
28.4 |
Not significant |
|
1 cycle of CPX-351 consolidation |
35.0 |
|
|
0 cycles of CPX-351 consolidation |
Not reached |
|
These results confirm the effectiveness of CPX-351 in sAML, including in patients with NPM1 mutations or ELN 2017 favorable risk. They also imply that allo-HSCT should be performed as soon as CR is achieved, and for patients who are ineligible for allo-HSCT further consolidation with CPX-351 is beneficial.
Key findings: Prognostic impact of TP53 mutations3
- CR rate after induction Cycle 1 was similar in patients with mutated TP53 vs TP53-wt but was lower in patients with mutated TP53 with concomitant CK vs TP53-wt (p < 0.05; Figure 2).
Figure 2. CR rate after induction Cycle 1 in patients evaluated for TP53 mutations*
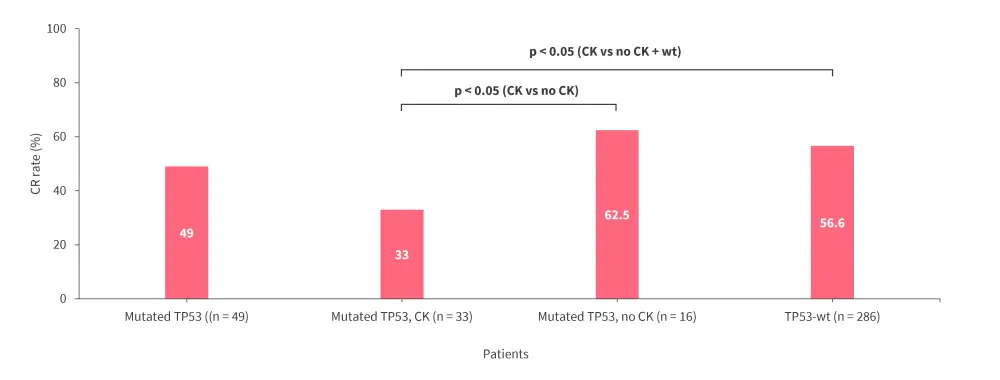
CK, complex karyotype; CR, complete remission; wt, wild-type.
*Data from Todisco.3
- Patients with mutated TP53 had a similar OS when compared with patients with TP53-wt; however, OS was poorer in patients with concomitant TP53 mutation and CK vs those with either TP53 mutation and no CK (p < 0.05) or those with TP53-wt (p < 0.05).
-
- Among patients with CK, the presence of TP53 was associated with a shorter OS compared with TP53-wt (p < 0.05).
- In patients who received allo-HSCT, those with concomitant mutated TP53 and CK had worse outcomes (Table 2; p < 0.05).
Table 2. Landmark analysis of patients alive and in CR at Day 90 according to TP53 mutational status and CK*
|
CK, complex karyotype; CR, complete remission; OS, overall survival; wt, wild-type. |
|
|
Patients who received allo-HSCT |
Median OS, months |
|---|---|
|
Mutated TP53, no CK + TP53-wt (n = 151) |
Not reached |
|
Mutated TP53, CK (n = 9) |
14.3 |
|
Mutated TP53 (n = 15) |
43.0 |
|
Mutated TP53, no CK (n = 6) |
Not reached |
This analysis confirms the efficacy of CPX-351 in patients with TP53-mutated AML and additionally found that concomitant CK had worse outcomes. Consolidation with allo-HSCT may improve survival outcomes in these patients.
Prognostic impact of high-risk mutations in elderly patients4
Study design and patient population
This single-center study included 80 patients with sAML, including t-AML, treated with CPX-351, with a median age of 70 years. Mutational analysis was performed using next-generation sequencing, and MRD testing was performed in all patients achieving CR with multicolor flow cytometry (MFC).
Key findings
- The most frequently observed mutations are shown in Figure 3.
Figure 3. Most frequently observed mutations*
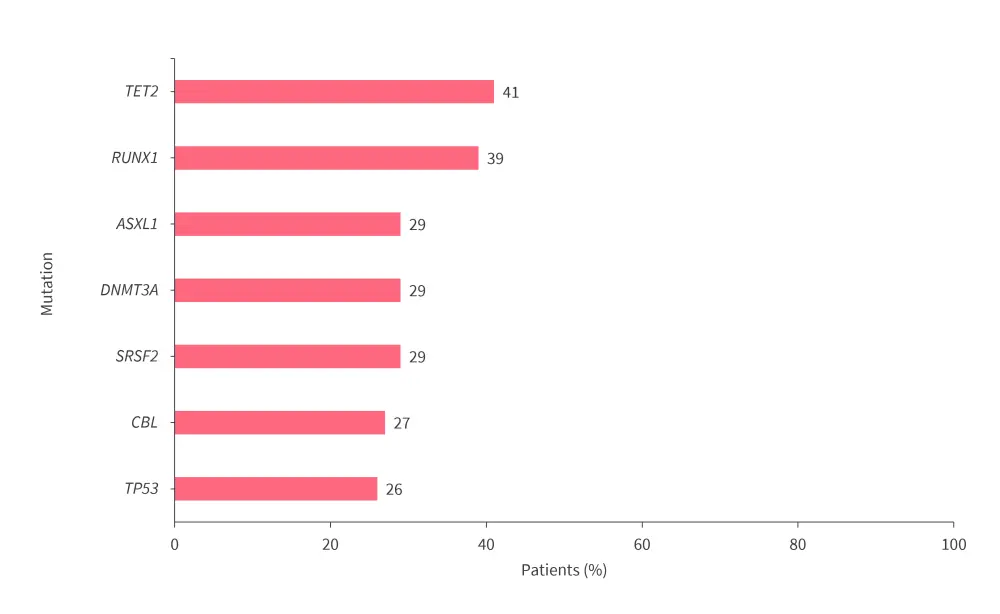
*Adapted from Riva.4
- 62.5% and 76% of patients showed molecular features linked to venetoclax or 7+3 chemotherapy resistance, respectively.
- After Cycle 1, 80% of patients achieved CR, of which 67% were MFC-MRD negative.
- At median follow-up of 39.3 months, median OS was 18 months, and 2-year OS rate was 31.8%.
- CR, MRD negativity, and OS were not affected by any of the high-risk mutations or high mutational burden, and OS was also not impacted by presence of a hypomethylating agent + venetoclax resistance profile; median OS was 13 vs 14 months for patients with vs without, respectively.
- Multivariate analysis showed that MRD negativity was the strongest prognostic factor for OS (p < 0.05).
- In landmark analysis of patients alive and in CR at Day 90, allo-HSCT (n = 23) within 3 months of achieving CR (n = 8) was associated with better outcomes vs no allo-HSCT (n = 41) or allo-HSCT after 3 months (n = 15; p < 0.03).
These results demonstrate that treatment with CPX-351 can induce MRD-negative CR in elderly patients with sAML, regardless of mutational burden or resistance to hypomethylating agent + venetoclax. In addition, early allo-HSCT consolidation was associated with improved long-term survival.
CPX-351 vs 7+3 in patients aged <60 years with AML-MR5
Study design and patient population
This study included 61 younger patients, aged 18–59 years, with AML-MR based on the 2022 WHO/ICC criteria, who were treated with CPX-351 as a first-line therapy, as part of the Myeloid Malignancy Association of Rapid Research Outcomes Working Group (MARROW). Outcomes were compared to a 1:2 matched retrospective cohort of 122 patients aged 18–59 years with AML-MR who received conventional 7+3 chemotherapy first-line therapy through protocols of the Cancer and Leukemia Group B (CALGB)/Alliance for Clinical Trials in Oncology (Alliance). Patients with mutated FLT3-ITD were excluded, and no patients in the Alliance cohort received allo-HSCT in first CR. The four subgroups of AML-MR were evenly matched between cohorts, with 25%, 30%, 72%, and 26% of patients with t-AML, antecedent MDS/chronic myelomonocytic leukemia (CMML), AML-MRC, and AML-MRmut, respectively.
Key findings
- In patients treated with CPX-351, the CR rate was 48% and composite CR (CRc) rate was 59%.
- In total, 18% and 19% of patients in the CPX-351 and 7+3 cohorts had TP53 mutations, respectively.
- In TP53-wt patients, CR rates (62% vs 55%; p = 0.60) and median OS (1.2 vs 1.2 years; p = 0.91) were similar between the CPX-351 and 7+3 cohorts.
- CRc rates and median OS were similar overall and in patients with AML-MRmut, regardless of treatment with CPX-351 or 7+3 (Figure 4).
- There were no significant differences in CRc rates and median OS across each AML-MR subgroup when comparing CPX-351 vs 7+3.
- In total, 25% of patients in the CPX-351 cohort received allo-HSCT in first CR, with a 3-year OS rate of 72%.
Figure 4. Comparison of A CRc rates and B median OS in patients with AML-MR and AML-MRmut treated with CPX-351 vs 7+3 chemotherapy*
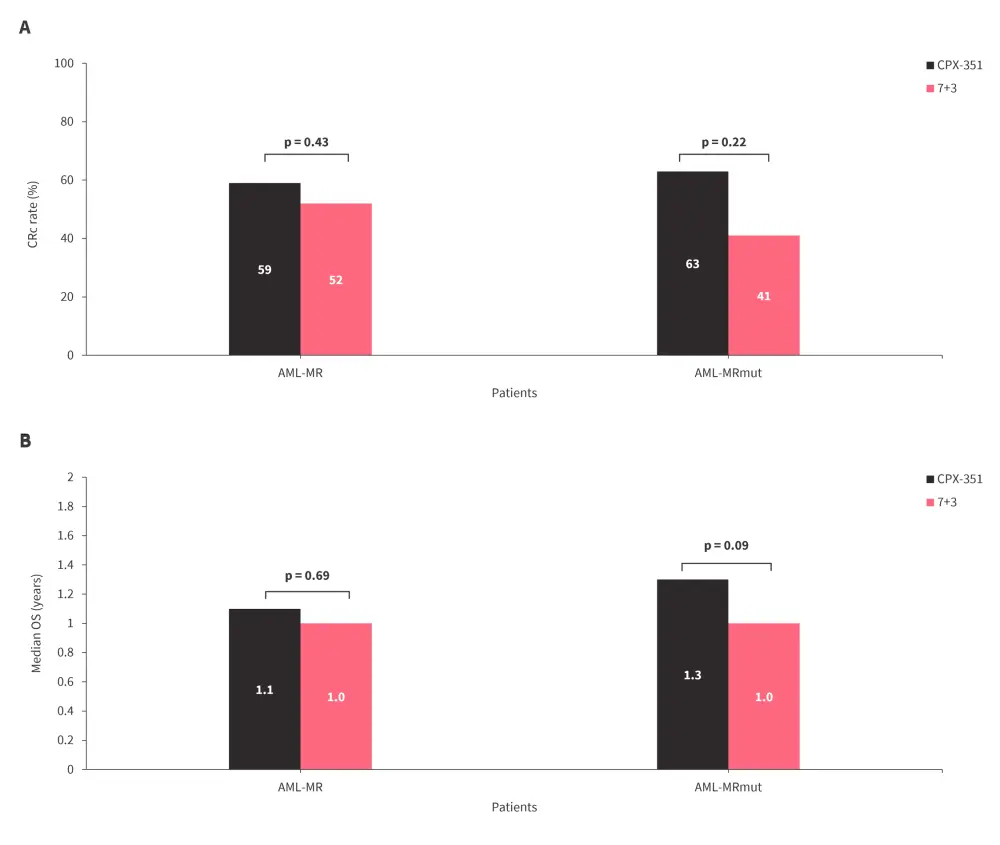
AML-MR, AML myelodysplasia-related; AML-MRmut, mutation-defined AML-MR; CRC, composite complete remission; OS, overall survival.
*Data from Peters.5
Based on these results, further clarification is needed to determine the impact of CPX-351 in the treatment of AML-MR and particularly in patients aged <60 years.
Conclusion
These real-world findings presented at EHA 2024 confirm the efficacy of CPX-351 in patients with sAML.1-5 First-line treatment with CPX-351 was associated with high response rates, regardless of mutational profile,3,4 although patients with mutated TP53 and concomitant CK did have poor outcomes.3 Several studies highlighted the survival benefit of allo-HSCT consolidation, particularly in patients with mutated TP53;1-4 however, in patients ineligible for allo-HSCT, consolidation with CPX-351 improved outcomes.2 Further analyses are needed to confirm whether treatment with CPX-351 confers a survival advantage over conventional 7+3 chemotherapy in patients aged <60 years within the updated subclass AML-MR.5 Taken together, these findings highlight the benefit of first-line treatment with CPX-351 in patients with sAML, and the importance of timely allo-HSCT in eligible patients in order to optimize outcomes.1–4
Your opinion matters
As a result of this content, I commit to reviewing real-world experiences with CPX-351 to guide my treatment of secondary AML in clinical practice.
This educational resource is independently supported by Jazz Pharmaceuticals. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

