All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
International Consensus Classification of AML
The revised fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues was published in 2016.1 Since then, further progress in the understanding of myeloid neoplasms and acute leukemias has prompted the need for a revision of previous classifications. Arber et al.2 recently published the new International Consensus Classification (ICC) of myeloid neoplasms and acute leukemia in Blood with the aim of updating current classifications. The published consensus classification incorporates morphologic, clinical, and genomic data to reflect the advancements that have led to a deeper understanding of these diseases. Below, we summarize the key points from the new ICC, in particular referring to acute myeloid leukemia (AML).
Methods2
A clinical advisory committee of global expert pathologists, hematologists, oncologists, and geneticists worked collaboratively to develop this ICC. Many of these authors worked on previous WHO editions; however, this ICC is no longer affiliated with the WHO.
Classification of AML2
The ICC AML categories are listed below in hierarchical order. The updated classifications retain many of the previously defined AML types with recurrent genetic abnormalities and include other genetic-related entities. The changes reflect a shift in focus to a more genetically-based classification.
- Acute promyelocytic leukemia (APL) with t(15;17)(q24.1;q21.2)/PML::RARA ≥10% blasts
- APL with other RARA rearrangements ≥10% blasts
- Including AMLs with t(1;17)(q42.3;q21.2)/IRF2BP2::RARA; t(5;17)(q35.1;q21.2)/NPM1::RARA; t(11;17)(q23.2;q21.2)/ZBTB16::RARA; cryptic inv(17q) or del(17) (q21.2q21.2)/STAT5B::RARA, STAT3::RARA; other genes rarely rearranged with RARA:TBL1XR1 (3q26.3), FIP1L1 (4q12),BCOR (Xp11.4)
- AML with t(8;21)(q22;q22.1)/RUNX1::RUNX1T1 ≥10% blasts
- AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/CBFB::MYH11 ≥10% blasts
- AML with t(9;11)(p21.3;q23.3)/MLLT3::KMT2A ≥10% blasts
- AML with other KMT2A rearrangements ≥10% blasts
- Including AMLs with t(4;11)(q21.3;q23.3)/AFF1::KMT2A; t(6;11)(q27;q23.3)/AFDN::KMT2A; t(10;11)(p12.3;q23.3)/MLLT10::KMT2A; t(10;11)(q21.3;q23.3)/TET1::KMT2A; t(11;19)(q23.3;p13.1)/KMT2A::ELL; t(11;19)(q23.3;p13.3)/KMT2A::MLLT1 (occurs predominantly in infants and children)
- AML with t(6;9)(p22.3;q34.1)DEK::NUP214 ≥10% blasts
- AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)/GATA2;MECOM(EVI1) ≥10% blasts
- AML with other MECOM rearrangements ≥10% blasts
- Including AMLs with t(2;3)(p11∼23;q26.2)/MECOM::?; t(3;8)(q26.2;q24.2)/MYC, MECOM; t(3;12)(q26.2;p13.2)/ETV6::MECOM; t(3;21)(q26.2;q22.1)/MECOM::RUNX1
- AML with other rare recurring translocations ≥10% blasts, such as
- AML with t(1;3)(p36.3;q21.3)/PRDM16::RPN1
- AML with t(3;5)(q25.3;q35.1)/NPM1::MLF1
- AML with t(8;16)(p11.2;p13.3)/KAT6A::CREBBP
- AML (megakaryoblastic) with t(1;22)(p13.3;q13.1)/RBM15::MRTF1
- AML with t(5;11)(q35.2;p15.4/NUP98::NSD1
- AML with t(11;12)(p15.4;p13.3)/NUP98::KMD5A
- AML with NUP98 and other partners
- AML with t(7;12)(q36.3;p13.2)/ETV6::MNX1
- AML with t(10;11)(p12.3;q14.2)/PICALM::MLLT10
- AML with t(16;21)(p11.2;q22.2)/FUS::ERG
- AML with t(16;21)(q24.3;q22.1)/RUNX1::CBFA2T3
- AML with inv(16)(p13.3q24.3)/CBFA2T3::GLIS2
- AML with t(9;22)(q34.1;q11.2)/BCR::ABL1 ≥20% blasts
- The category of myelodysplastic syndromes (MDS)/AML will not be used for AML with BCR::ABL1 due to its overlap with progression of chronic myeloid leukemia, BCR::ABL1-positive
- AML with mutated NPM1 ≥10% blasts
- AML with in-frame bZIP CEBPA mutations ≥10% blasts
- The previous category of AML with biallelic CEBPA mutations has been changed due to several studies indicating that the presence of in-frame bZIP mutations of CEBPA defines the prognostic entity with a unique gene expression profile. This category is updated to include this abnormality without the requirement of biallelic mutations
- AML and MDS/AML with mutated TP53 10–19% blasts (MDS/AML) and ≥20% blasts (AML)
- AML and MDS/AML with myelodysplasia-related gene mutations 10–19% blasts (MDS/AML) and ≥20% blasts (AML)
- Defined by mutations in ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, or ZRSR2
- AML and MDS/AML with myelodysplasia-related cytogenetic abnormalities 10–19% blasts (MDS/AML) and ≥20% blasts (AML)
- Defined by detecting a complex karyotype (≥3 unrelated clonal chromosomal abnormalities in the absence of other class-defining recurring genetic abnormalities), del(5q/t(5q)/add(5q), −7/del(7q), +8, del(12p)/t(12p)/add(12p), i(17q), −17/add(17p) or del(17p), del(20q), and/or idic(X)(q13) clonal abnormalities
- AML not otherwise specified (NOS) 10–19% blasts (MDS/AML) and ≥20% blasts (AML)
- Myeloid sarcoma
The genetic disease groups named after a single abnormality rarely show overlap. While TP53 mutations may overlap, their presence is usually an indication of an adverse prognosis. Single gene mutations or gene fusions take precedence over myelodysplasia-related gene mutations and myelodysplasia-related cytogenetic abnormalities. However, these findings may impact prognosis and should be noted. Cases that do not fall under any of the categories listed should be classified as AML NOS.
Diagnostic qualifiers2
The focus on genetic factors in the updated classification of AML has led to the elimination of the previous standalone categories of therapy-related myeloid neoplasms and AML with myelodysplasia-related changes (AML-MRC). Prior therapy, antecedent myeloid neoplasms, and underlying germline genetic predispositions are now used as diagnostic qualifiers rather than separate categories of AML (Figure 1).
Figure 1. Diagnostic qualifiers following a specific MDS, AML, or MDS/AML diagnosis*
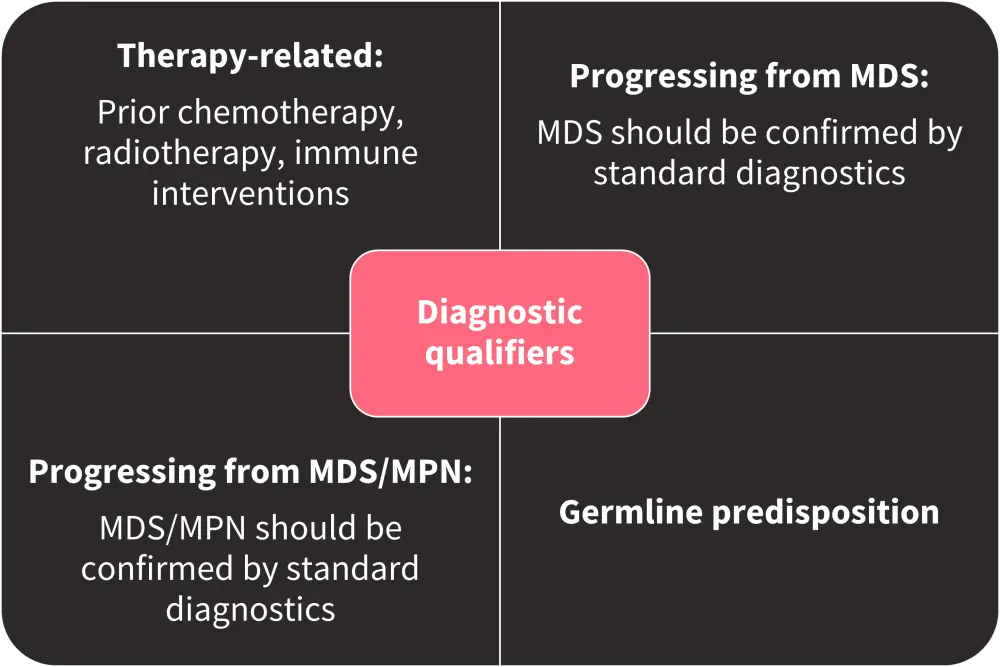
AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; MPN, myeloproliferative neoplasms.
*Data from Arber, et al. 2022.2
†Lymphoblastic leukemia/lymphoma may also be therapy related, and the association should be noted in the diagnosis.
AML-MRC2
The prior category of AML-MRC was used to identify patients with a worse prognosis compared to patients with AML NOS; however, due to the association of multilineage dysplasia in a subset of patients with low-risk gene mutations, and an overlap of features between AML-MRC and therapy-related AML, a need for further molecular refinement was necessary.
AML with mutated TP53 is now recognized as a separate disease entity and is typically associated with complex cytogenetic abnormalities and a very poor prognosis, as AML with mutated TP53 represents a distinctly aggressive form of AML. Any pathogenic TP53 mutation with variant allele frequency >10% is sufficient for diagnosis (Table 1).
Mutations defined as myelodysplasia-related mutations that are highly associated with secondary AML arising from prior myeloid neoplasia have also been identified. Myelodysplasia-related mutations are associated with a similarly adverse prognosis to de novo AML, previously classified as AML NOS. With these changes to classification, AML-MRC is eliminated while AML with myelodysplasia-related cytogenetic abnormalities is retained. The two new categories described above—mutated TP53 and AML with myelodysplasia-related gene mutations—are added.
Table 1. AML with mutated TP53*
|
AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; TP53, tumor protein P53 gene; VAF, variant allele frequency. |
|||
|
Type |
Cytopenia |
Blasts |
Genetics |
|---|---|---|---|
|
MDS/AML with mutated TP53 |
Any |
10–19% bone marrow or blood blasts |
Any somatic TP53 mutation (VAF >10%) |
|
AML with mutated TP53 |
Not required |
≥20% bone marrow or blood blasts or meets criteria for pure erythroid leukemia |
Any somatic TP53 mutation (VAF >10%) |
Blast percentage2
Recurrent genetic abnormalities (including gene mutations) with ≥10% blasts in the blood or bone marrow can now be diagnosed as AML. Cases of MDS with 10–19% blasts are categorized as MDS/AML with particular genetic features such as mutated TP53, myelodysplasia-related gene mutations, myelodysplasia-related cytogenetic abnormalities, or NOS. The blast threshold remains at ≥20% for the rest of the AML categories.
Conclusion
The ICC update to the classification of AML reflects the advancements in the understanding of AML biology, experience in clinical practice, and the results of the latest clinical trials. Classification now places more emphasis on genetic factors while moving some previous categories, such as therapy-related AML, to diagnostic qualifiers. The previous category of AML-MRC has also been removed in an attempt to better predict prognosis based on genetic characteristics. Another major change in the updated classification comes in the form of lowering the blast threshold for several AML categories. While previously a blast percentage of ≥20% was required, several cases including specific features and 10–19% blasts are now classified as MDS/AML, recognizing the diagnostic continuum between AML and MDS.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

