All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Symposium | Implications for clinical practice: Managing AML with FLT3-ITD and -TKD mutations
Featured:
Do you know... What is the impact of MRD on post-transplant outcomes in patients with FLT3-mutated AML?
The AML Hub held a virtual symposium on November 19, 2025, titled Understanding the differences between FLT3-ITD and -TKD mutations in AML: Implications for clinical practice. Here, we share a presentation from the symposium by Gail J. Roboz, Weill Cornell Medicine, New York, US, in which she discussed the management of patients with acute myeloid leukemia (AML) with FLT3-internal tandem duplication (ITD) and FLT3-tyrosine kinase domain (TKD) mutations in clinical practice.
Symposium | Implications for clinical practice: Managing AML with FLT3-ITD and -TKD mutations
Symposium | Implications for clinical practice: Managing AML with FLT3-ITD and -TKD mutations
Roboz reflected on whether 7+3 regimens are the most appropriate approach for older patients with FLT3-mutated AML, and reviewed considerations for using targeted FLT3 inhibitor therapies in combination with standard intensive chemotherapy. She then highlighted the importance of measurable residual disease (MRD) assessment in guiding treatment decisions before and after allogeneic hematopoietic stem cell transplantation (allo-HSCT), and the potential of triplet therapies for the treatment of patients with FLT3m AML.
Key points
- In clinical practice, standard of care treatment for patients with FLT3m AML is a FLT3 inhibitor in combination with a standard 7+3 backbone; however, clinical trials investigating these regimens have primarily mainly included younger patient populations.
- For instance, the RATIFY trial investigated midostaurin with 7+3 in patients aged 18–60 years with newly diagnosed (ND) FLT3m AML,1 and the QuANTUM-First trial investigated quizartinib with 7+3 in patients aged 18–75 years with ND FLT3-ITD AML.2
- Ten-year follow-up data from the RATIFY trial indicate that improvements in survival were primarily driven by allo-HSCT; the median overall survival (OS) in patients who underwent transplant in first complete remission was not evaluable vs 24.6 months in those who did not (p < 0.0001).3
- Therefore, there is still an unmet need to identify the most appropriate approach for patients not eligible for transplant, such as older patient populations.
- As understanding of AML develops, treatment algorithms are becoming increasingly complex. Given that MRD is a key prognostic factor in AML,4–7 algorithms are now tailored according to MRD status as well as mutation type and risk classification.8
- An important consideration for MRD assessment is the choice of assay; conventional polymerase chain reaction (PCR) assays used for FLT3m AML diagnosis are not as helpful for FLT3-ITD MRD detection due to their limit of sensitivity (~1%).5
- The PCR next-generation sequencing (PCR-NGS) assay was developed specifically for the QuANTUM-First trial and utilizes ultra-deep sequencing to identify FLT3-ITD MRD with high sensitivity and specificity (Figure 1).5,7
Figure 1. High-sensitivity MRD assessment for FLT3-ITD AML4–7,9
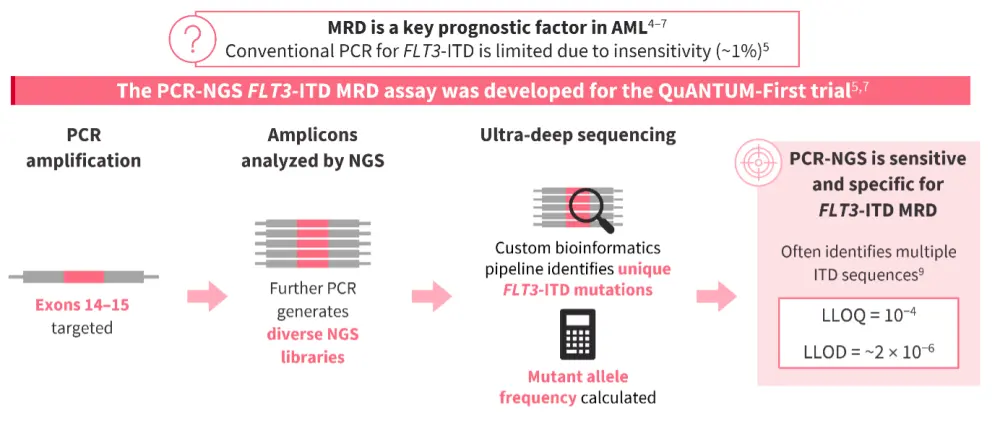
- The impact of MRD has been demonstrated in the post-transplant maintenance setting in the phase III MORPHO trial (NCT02997202) of gilteritinib in patients with FLT3-ITD AML following HSCT, where any detectable level of MRD was associated with poorer outcomes compared with MRD negativity.10
- In the placebo arm, patients with MRD-positive AML were found to relapse quickly following HSCT. Of patients with MRD positivity, 42.2% relapsed vs 13.4% of patients with MRD negativity.10
- Gilteritinib maintenance reduced the risk of post-HSCT relapse in patients with FLT3m MRD positivity either before or after HSCT.11
- For older patients with FLT3m AML who are not eligible for transplant, non-intensive regimens of venetoclax plus a hypomethylating agent (HMA) have been investigated.8
- Data have demonstrated that survival benefit with venetoclax + azacitidine is primarily driven by outcomes in the subgroup of patients with FLT3-TKD AML (median OS, 19.2 months vs 9.9 months in patients with FLT3-ITD AML).12
- An alternative proposed strategy for these patients is to add a FLT3 inhibitor to the non-intensive backbone.13
- A retrospective analysis of outcomes with quizartinib or gilteritinib + venetoclax and an HMA indicated that these triplet regimens may improve relapse-free survival and OS in patients with FLT3-ITD or -TKD AML.13
- Trials are ongoing investigating FLT3 inhibitor combinations with standard chemotherapy, as well as with less intensive regimens for those patients who are unable to tolerate standard chemotherapy.14–24
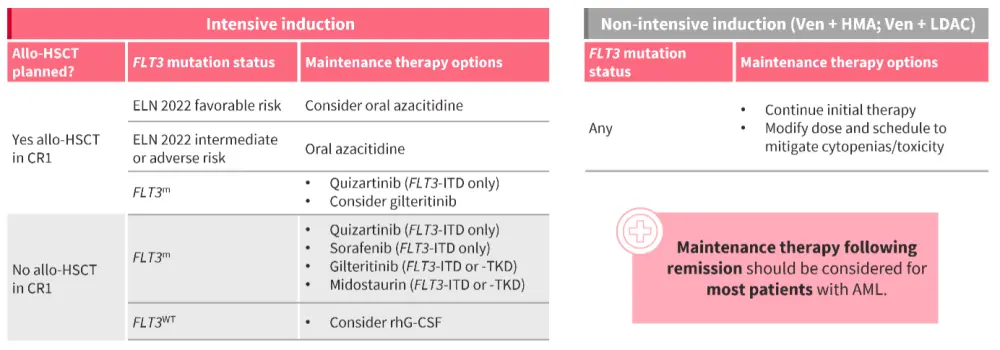
- For the majority of patients, maintenance therapy following remission should be considered, and tailored depending on whether they will undergo allo-HSCT, their risk classification, and mutation status (Figure 2).25
Figure 2. Maintenance therapy should be considered for the majority of patients with AML25
This educational resource is independently supported by Daiichi Sankyo. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Gail J. Roboz
Gail J. Roboz




