All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Recent advancements in the use of menin inhibitors for AML: Highlights from EHA 2024
Do you know... In the phase II AUGMENT trial, the primary endpoint of complete remission (CR)/CR with partial hematologic recovery (CRh) with revumenib was met. What percentage of patients achieved CR/CRh?
The interaction between menin and KMT2A plays an important role in aberrant transcription and leukemogenesis in acute myeloid leukemia (AML), as previously covered on the AML Hub. Therefore, menin inhibitors represent an important class of therapeutic agents for patients with AML, particularly those with mutated NPM1 (NPM1m) or KMT2Arearrangements (KMT2Ar) leading to overexpression of HOXA9 and MEIS1 genes.1-4
Below, we summarize six presentations from the European Hematology Association (EHA) 2024 Hybrid Congress, by Issa,1 Zeidner,2 Wei,3 Daver,4 Hertlein,5 and Mitra,6 on menin inhibitors that are currently in development for the treatment of AML.
Revumenib (SNDX-5613) monotherapy in patients with relapsed/refractory AML with KMT2Ar (AUGMENT-101)1
Revumenib, a selective, small-molecule inhibitor of the menin-KMT2A interaction (Figure 1), is in development for the treatment of AML with KMT2Ar and NPM1m.
Figure 1. Mechanism of action of revumenib*
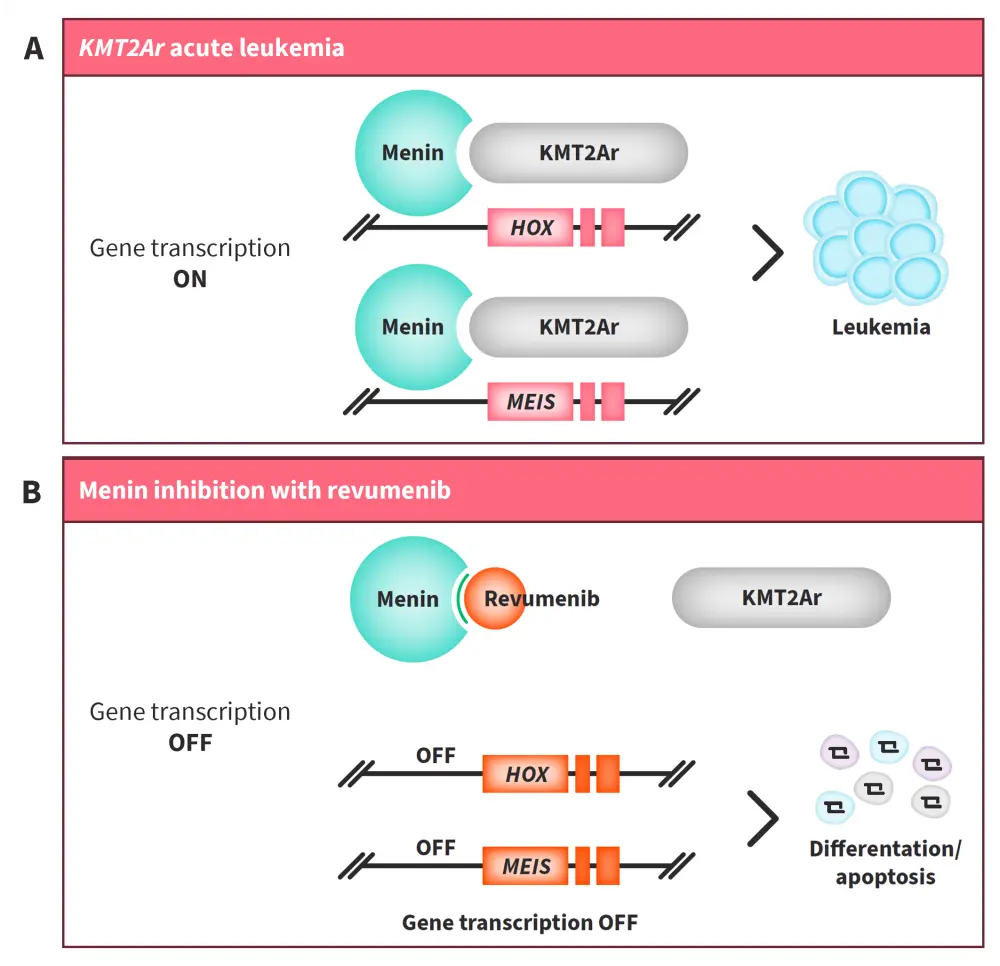
A Rearrangements in KMT2A result in overexpression of HOX and MEIS genes, which blocks hematopoietic differentiation and leads to promotion of leukemia cells. Menin is crucial for the KMT2A protein complex to bind to the HOX gene. B Revumenib, a potent, selective, small-molecule inhibitor of the menin-KMT2A interaction, downregulates HOX and MEIS transcription, leading to hematopoietic differentiation and an antileukemic effect.
AML, acute myeloid leukemia; KMT2Ar, histone-lysine-N-methyltransferase 2A rearrangement; NPM1m, mutated NPM1.
*Adapted from Issa1 and data from Issa et al.7
Study design and patient population
In the AUGMENT-101 phase II trial (NCT04065399; Figure 2), 94 heavily pre-treated patients were included in the safety population (median age, 37 years; female, 56%) and 57 in the efficacy population (median age, 34 years; female, 58%) at the data cut-off of July 24, 2023.
Figure 2. AUGMENT-101 study design*
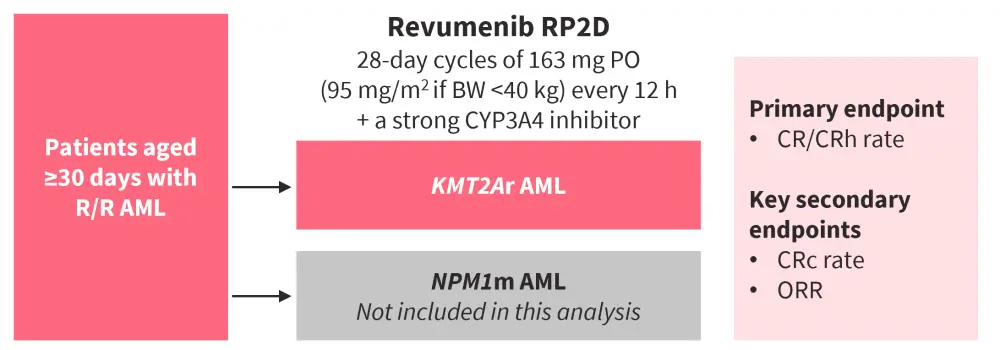
AML, acute myeloid leukemia; BW, body weight; CR, complete remission; CRc, composite CR (CR/CRh/CRp/CRi); CRh, CR with partial hematologic recovery; CRi, CR with incomplete hematologic recovery; CRp, CR with incomplete platelet recovery; KMT2Ar, KMT2A rearrangement; NPM1m, mutated NPM1; ORR, overall response rate; PO, oral administration; R/R relapsed/refractory; RP2D, recommended phase II dose.
*Adapted from Issa.1 Data cut-off: July 24, 2023.
Key findings
- At a median follow-up of 6.1 months, the primary efficacy endpoint was met, with 23% patients achieving complete remission (CR)/CR with partial hematologic recovery (CRh; p = 0.0036; Figure 3A).
- Among responders, 7/10 evaluable patients achieved minimal residual disease negativity and 6/14 evaluable patients proceeded to hematopoietic stem cell transplantation (HSCT).
- Median time to CR/CRh was 1.87 months.
- Median duration of response was 6.4 months.
- Median overall survival was 8 months (95% CI, 4.1–10.9).
- Responses were observed across different subgroups and type of KMT2A rearrangement (Figure 3B).
Figure 3. Response rates observed with revumenib A in the overall efficacy population and B stratified by subgroups and KMT2A rearrangement*
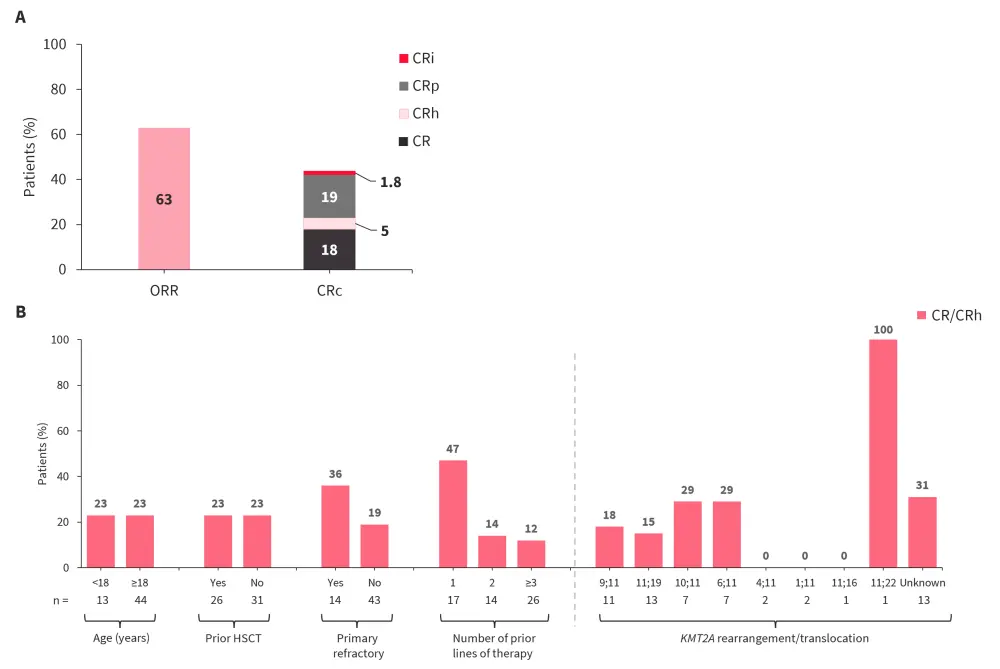
CR, complete remission; CRc, composite CR (CR/CRh/CRp/CRi); CRh, CR with partial hematologic recovery; CRi, CR with incomplete hematological recovery; CRp, CR with incomplete platelet recovery; HSCT, hematopoietic stem cell transplantation; ORR, overall response rate.
*Data from Issa.1
- The most common Grade ≥3 treatment-emergent adverse events (TEAEs) occurring in ≥10% patients were febrile neutropenia (37%), anemia (18%), neutropenia (16%), leukopenia (16%), thrombocytopenia (15%), differentiation syndrome (DS; 16%), corrected QT (QTc) prolongation (14%), sepsis (12%), and hypokalemia (11%).
- There were no treatment discontinuations due to DS, QTc prolongation, or cytopenias.
Revumenib in combination with azacitidine and venetoclax in newly diagnosed AML with NPM1m or KMT2Ar (Beat AML)2
Venetoclax plus azacitidine is an important combination therapy for older patients with AML; however, it is still limited by poor long-term outcomes and relapse rates in patients with NPM1m or poor risk cytogenetics. Hence, the combination of venetoclax and azacitidine with revumenib is currently under investigation in patients with newly diagnosed (ND) AML with NPM1m or KMT2Ar.
Study design and patient population
One substudy of the Beat AML master trial is an open-label phase Ib dose escalation and expansion trial (NCT03013998) evaluating the safety and recommended dose of revumenib in combination with azacitidine and venetoclax in patients with ND AML with NPM1m or MLL rearrangement (MLLr), who are unable to undergo intensive induction therapy (Figure 4).2,8
Two dose levels (DLs) of revumenib were assessed:
- DL1a: 113 mg orally every 12 hours for 28 days
- DL2a: 163 mg orally every 12 hours for 28 days
Figure 4. Beat AML substudy design of revumenib + venetoclax + azacitidine in ND NPM1m or KMT2Ar AML*
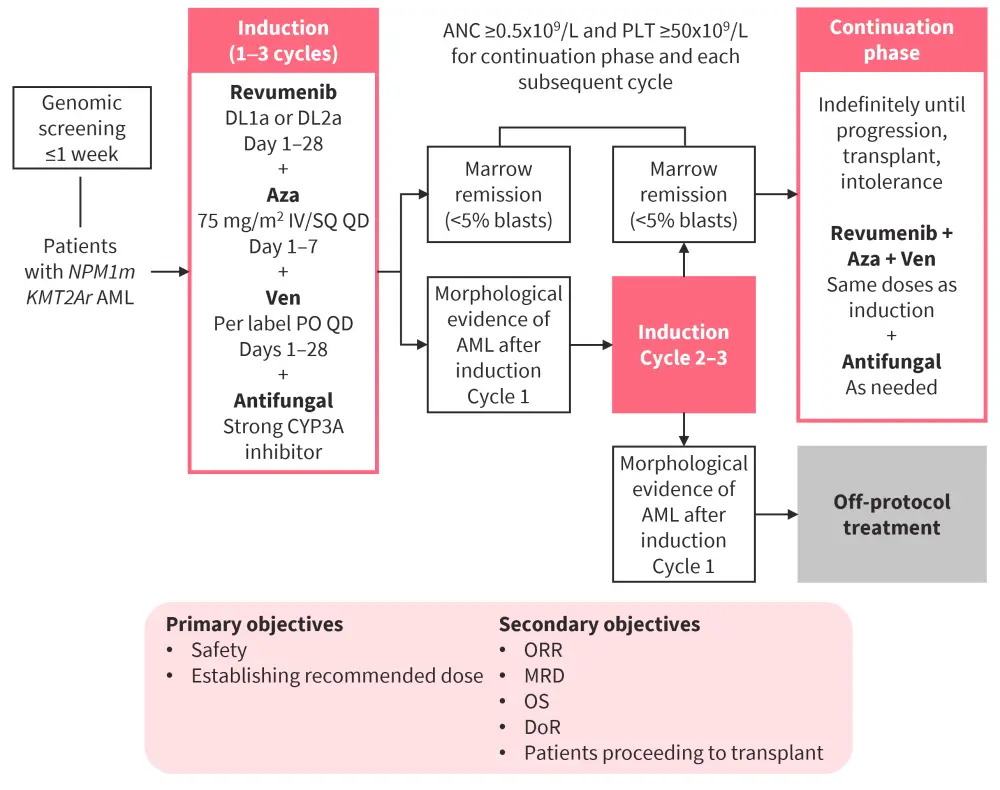
ANC, absolute neutrophil count; AML, acute myeloid leukemia; Aza, azacitidine; DLT, dose-limiting toxicity; DoR, duration of response; IV, intravenous; KMT2Ar, KMT2A rearrangement; MRD, minimal residual disease; ND, newly diagnosed; NPM1m, mutated NPM1; ORR, overall response rate; OS, overall survival; PLT, platelets; PO, oral administration; QD, once a day; SQ, subcutaneous; Ven, venetoclax.
*Adapted from Zeidner.2
A total of 26 patients aged ≥60 years were included (7 in escalation DL1a, 6 in escalation DL2a, and 13 in expansion DL2a).
- Median age was 70 years, with 34.6% patients aged ≥75 years.
- 65.4% of patients had NPM1m AML, while 34.6% had KMT2Ar AML.
Key findings
- Revumenib in combination with venetoclax and azacitidine was administered safely at both DLs to patients aged≥60 years newly diagnosed with NPM1m or KMT2Ar AML.
- There was no maximum tolerated dose.
- The most common Grade 1–2 non-hematologic AEs across both DLs were constipation (54%), nausea (54%), vomiting (46%), QT prolongation (46%), hyponatremia (42%), and diarrhea (38%).
- QTc prolongation and DS (observed in 15% patients) were self-limiting and resolved without complications.
- The median induction cycle length was 36 days.
- 80% of patients experienced cytopenias resulting in a delay in starting the continuation phase.
- There were no differences in time to initiation of continuation between revumenib dose levels, suggesting that cytopenias were related to venetoclax and not revumenib.
- Of 24 evaluable patients, 96% patients achieved composite CR (CR/CRh/CR with incomplete hematologic recovery [CRi]) and 100% achieved overall response (CR/CRh/CRi/morphological leukemia-free state).
- 92% of patients achieved minimal residual disease negativity.
Bleximenib (JNJ-75276617) in combination with venetoclax and azacitidine in KMT2Ar or NPM1m R/R AML3
Findings from a phase I study (NCT04811560) of bleximenib monotherapy in patients with R/R AML with KMT2Ar or NPM1m have previously been published on the AML Hub. Below, we summarize results from Cohort A3 of an ongoing phase Ib trial (NCT05453903) of bleximenib in combination with venetoclax and azacitidine in KMT2Ar or NPM1m R/R AML (Figure 5).
Study design and patient population
Figure 5. A Study design and B dosing schedule of a phase Ib trial of bleximenib + Ven + Aza in KMT2Ar or NPM1m R/R AML*
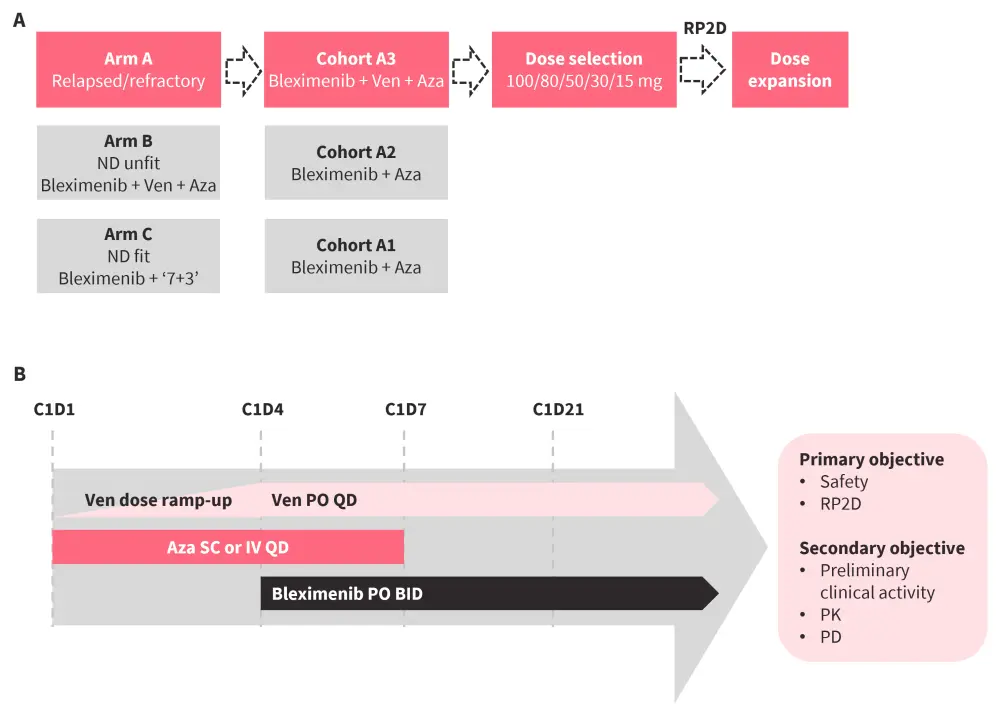
Aza, azacitidine, BID, twice a day; C, cycle; D, day; IV, intravenous; ND, newly diagnosed; PD, pharmacodynamic; PK, pharmacokinetic; PO, oral administration; QD, once a day; RP2D, recommended phase II dose; R/R relapsed/refractory; SC, subcutaneous; Ven, venetoclax.
*Adapted from Wei.3 Data cut off: May 7, 2024
A total of 60 patients were included, with a median age of 60 years and 55% female.
- Patients were treated with a median of two prior lines of therapy, including 32% with HSCT and 48% with venetoclax.
- A total of 30 patients with AML had KMT2Ar and 30 had NPM1m.
- FLT3 was the most common co-mutation, primarily observed in patients with NPM1m AML, while TP53 was predominant in patients with KMT2Ar AML. Other co-mutations included DNMT3A, TET2, IDH2, IDH1,KRAS, and RAD21.
Key findings
- Bleximenib in combination with venetoclax and azacitidine was well tolerated in the intent-to-treat safety set (N = 60).
- DS was observed in 2 patients (Grade 3 at 100 mg twice a day [BID] and Grade 5 at 50 mg BID)
- No TEAEs of QTc prolongation or tumor lysis syndrome were reported.
- All grades TEAEs reported in ≥10% of patients were nausea (55%), vomiting (42%), febrile neutropenia (38%), thrombocytopenia (38%), anemia (35%), neutropenia (30%), diarrhea (25%), and pyrexia (25%).
- Recommended phase II dose (RP2D) was not yet determined.
- The combination demonstrated efficacy in 34 patients, including 13 patients with KMT2Ar and 21 with NPM1m, and in patients with prior venetoclax exposure (Figure 6).
- All patients experienced a reduction in leukemic burden; bone marrow blast reduction of ≥50% was observed in 93% of patients.
Figure 6. Preliminary clinical activity*
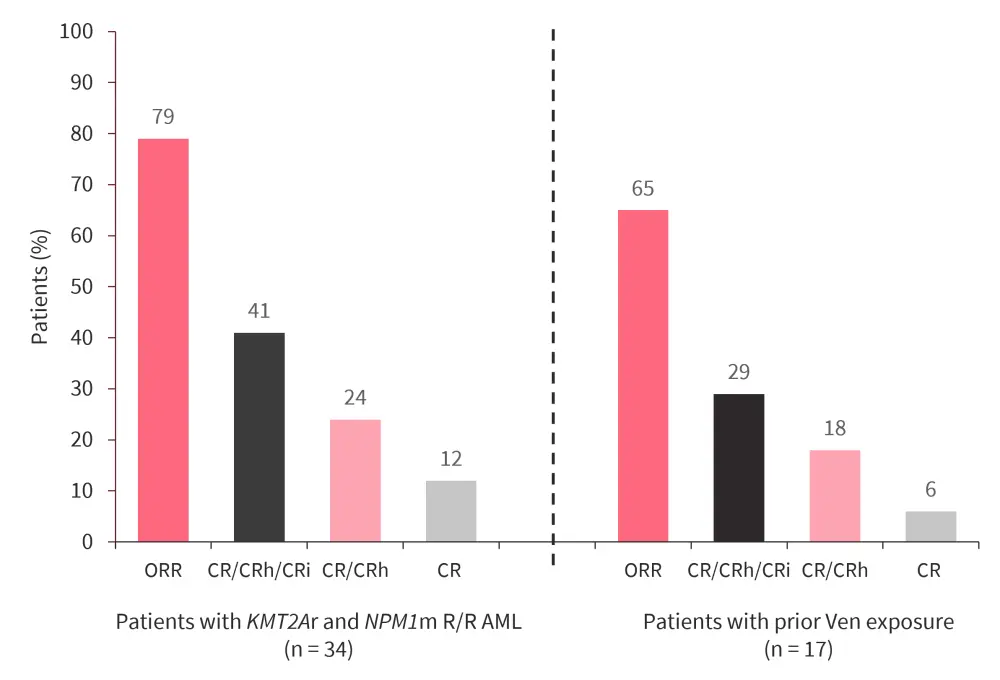
CR, complete remission; CRh, CR with partial hematologic recovery; CRi, CR with incomplete hematologic recovery; KMT2Ar, KMT2A rearrangement; NPM1m, mutated NPM1; ORR, overall response rate; Ven, venetoclax.
*Data from Wei.3
DSP-5336 in patients with R/R AML4
Study design and patient population
A first-in-human phase I/II study (NCT04988555) assessed DSP-5336 in patients with R/R MLL rearrangement (MLLr) or NPM1m AML (Figure 7). Among the 57 patients included, median age was 63 years and 57.9% were female. A total of 87.7% of patients had previously received treatment with venetoclax.
Figure 7. Study design of a phase I/II study assessing DSP-5336 in patients with R/R MLLr or NPM1m AML*
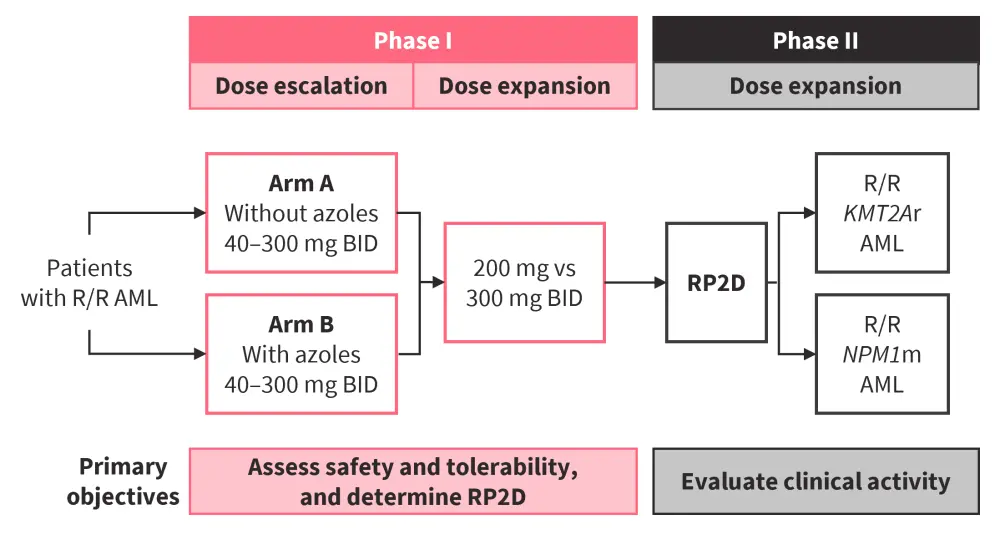
AML, acute myeloid leukemia; BID, twice a day; MLLr, MLL rearrangement; NPM1m, mutated NPM1; RP2D, recommended phase II dose; R/R, relapsed/refractory.
*Adapted from Daver.4 Data cut off: May 7, 2024.
Key findings
- DSP-5336 was well tolerated, with no dose-limiting toxicities, treatment-related deaths, cardiac toxicity, or discontinuations reported.
- DS was observed in 5.7% patients, with no prophylaxis required; no mortality or permanent discontinuation due to DS were reported.
- Non-hematologic AEs were consistent with an R/R AML population.
- Any grade hematologic AEs reported in ≥10% of patients were febrile neutropenia (21.1%), leukocytosis (17.5%), anemia (15.8%), thrombocytopenia (15.8%), and neutropenia (12.3%).
- In patients naïve to menin inhibitors who received a therapeutic dose of ≥140 mg BID (intent-to-treat population; n = 21), objective response rate was 57%, composite CR rate was 33%, and CR/CRh rate was 24%.
- Median time to CR/CRh was 1.4 months.
- Blast reductions were observed across all DLs.
- Pharmacokinetic data showed no large (>2-fold) drug–drug interactions with azoles, and only minimal accumulation with repeated dosing.
- Compared with a non-target population, the target population showed rapid decreases in stemness markers (HOXA9, MEIS1, and PBX3) and an increase in the differentiation marker CD11b from baseline (Figure 8).
Figure 8. Changes in markers with DSP-5336*
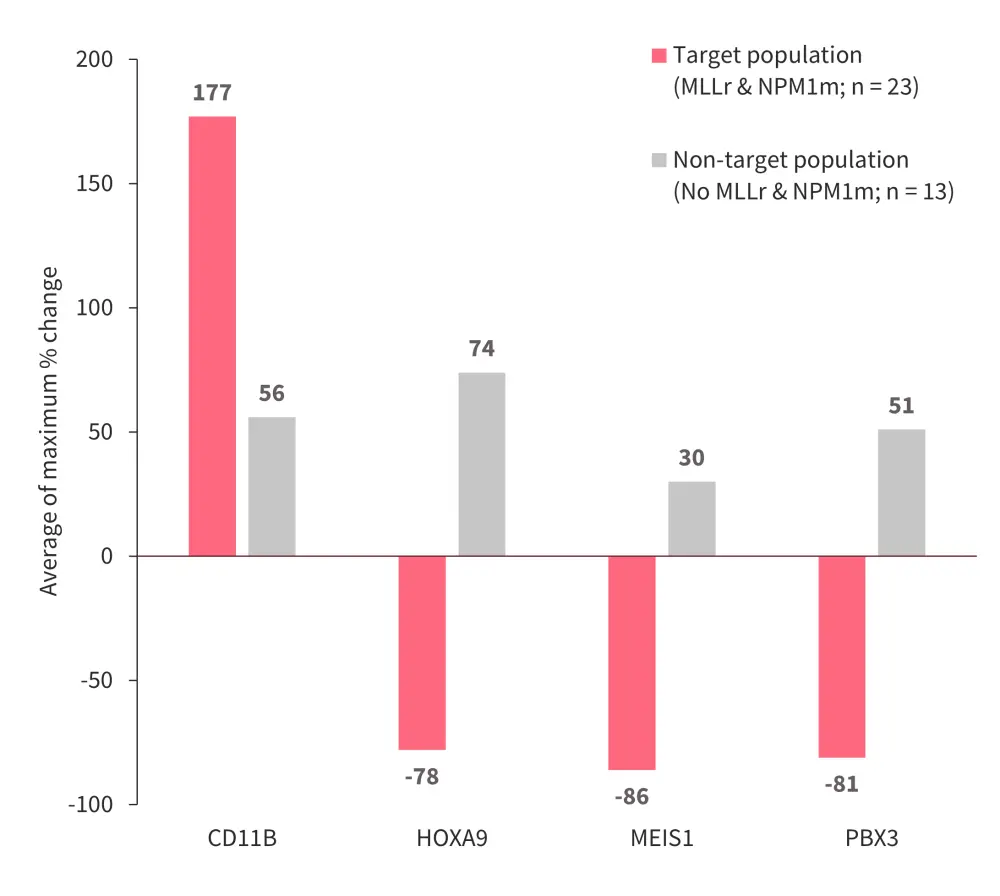
MLLr, MLL rearrangement; NPM1m, mutated NPM1.
*Data from Daver.4
Balomenib (ZE63-0302) preclinical studies5
The therapies targeting the menin-KMT2A interaction have several limitations, including drug-related cardiac toxicities (such as QTc prolongation) and hotspot mutations in MEN1 at the drug-binding interface. These resistance mutations (e.g., M327 and G331) hinder the interaction of menin-inhibitors with the target residue W346, resulting in loss of their therapeutic potential. Balomenib, a next-generation menin inhibitor, is designed to form an energetically favorable conformation enabling effective target interaction while avoiding W346, thereby addressing cardiotoxicity and reducing the risk of resistance mutations associated with menin inhibitors.
Methods
- QTc prolongation and cardiotoxicity were analyzed using rabbit Purkinje fiber assays.
- In vivo studies were performed on a MOLM-13 cell line xenograft model.
Key findings
- Balomenib exhibited a similar cytotoxic concentration (CC50) to revumenib in MV4-11 (15 vs 14), MOLM-13 (80 vs 60), and HEK293 (>3,000 in both) cell line models.
- Balomenib showed favorable pharmacokinetics in mice and human primates.
- In a MOLM-13 cell line xenograft model, balomenib monotherapy BID exhibited good efficacy compared with revumenib.
- In an 11-day toxicity study in a canine model:
- A balomenib single dose of 100 mg/kg did not show any effect on QTc.
- Balomenib ≤150 mg/kg BID showed no electrocardiogram changes.
- In rabbit Purkinje Fibers, no action potential prolongation was observed with balomenib ≤10 μm.
- The combination of balomenib with either ionitoclax (BCL2 inhibitor) or iomonitinib (FLT3 inhibitor) exhibited synergy and improved survival.
Ziftomenib in patients with R/R AML6
Ziftomenib is currently being investigated in a phase I/II study (KOMET-001 study; NCT04067336); interim findings have previously been published on the AML Hub. Below, we summarize information on the pharmacology and pharmacokinetic profile of ziftomenib.
Methods
- Samples were collected in Part 1a (dose escalation) and Part 1b (dose validation) of the KOMET-001 study, and subjected to non-compartmental analysis, population pharmacokinetic (popPK), and physiological-based PK (PBPK) modeling.
Key findings
- A linear, dose proportional increase in exposure up to the RP2D of 600 mg ziftomenib was observed.
- Coadministration of ziftomenib with azoles, which are strong (posaconazole and voriconazole) or moderate (fluconazole, isavuconazole, and isavuconazonium) CYP3A4 inhibitors, showed non-meaningful increases in exposure (AUC and Cmax) compared with administration without azoles.
- In popPK modeling, mild or moderate hepatic or renal impairment had no effect on ziftomenib clearance, compared with normal hepatic or renal function.
- PBPK modeling showed that ziftomenib exhibited no clinically meaningful drug–drug interaction with strong and moderate CYP3A4 inhibitors or the CYP3A4 substrates midazolam and venetoclax, hence no dose adjustments are needed when ziftomenib is co-administered with these agents.
Conclusion
These presentations demonstrate the potential of menin inhibitors in the treatment of AML. Revumenib was well tolerated and effective in both pediatric and adult patients with R/R KMT2Ar or NPM1m AML, and was also found to be safe and effective in combination with venetoclax and azacitidine in older patients with AML. Bleximenib, in combination with venetoclax and azacitidine, and DSP-5336 were seen to be well tolerated and effective, although their RP2D is yet to be determined. Balomenib demonstrated efficacy and a manageable safety profile in preclinical models, and showed synergy when combined with BCL2 and FLT3 inhibitors. At the RP2D, ziftomenib had a stable pharmacokinetic profile, with no significant drug interactions with potential combination therapies.
These menin inhibitors in early-stage clinical development, both as monotherapy or in combinations, show promising initial results, although further studies are warranted to determine their full therapeutic potential in patients with AML.
Your opinion matters
As a result of this content, I commit to reviewing the latest data on menin inhibitors to guide my treatment of relapsed/refractory AML in clinical practice.
This educational resource is independently supported by Syndax. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



