All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
The potential of menin-KMT2A inhibitors in the treatment of AML
Do you know... The interaction between menin and KMT2A is a key driver of leukemogenesis, and menin-KMT2A inhibitors are a promising approach for patients with KMT2A-rearranged AML. Which genes are downregulated following inhibition of the menin-KMT2A complex?
Rearrangements in the KMT2A (KMT2Ar) and mutations in the NPM1 gene occur in approximately 9–15% and 30%, respectively, of adult patients diagnosed with acute myeloid leukemia (AML), and are associated with poor outcomes.1 Patients with KMT2Ar are often refractory to standard treatments, leading to relapse, with a median overall survival rate (OS) of 2.4 months.3 Therefore, targeting the menin-KMT2A complex is a promising new approach to treatment in this population.
During the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, preliminary results from early phase trials of menin inhibitors were presented. The AML Hub is pleased to summarize presentations from Jabbour on JNJ-75276617,2 Aldoss on revumenib monotherapy, 3 Issa on the combination of revumenib + decitabine + cedazuridine plus venetoclax, 4 Basyal on menin inhibition-induced proteomic alterations,5 and Balasubramanian and Goldberg on ziftomenib combinations in patients with AML.6,7
JNJ-75276617 in patients with KMT2A-rearranged or NPM1-mutated relapsed/refractory (R/R) acute leukemia2
Initial analysis from an ongoing phase I, multicenter, open-label trial (NCT04811560) assessing the safety and efficacy of JNJ-75276617 in patients with R/R AML harboring KMT2A or NPM1 alterations.
- Primary endpoints were recommended phase 2 dose (RP2D), safety, and tolerability
- Secondary endpoints included pharmacokinetics and pharmacodynamics
- Dosing started with a single dose daily, followed by two doses daily after step-up dosing
- A total of 86 heavily pretreated patients were included, with a median age of 59 years and 60% refractory to venetoclax
Safety
The most common treatment-emergent adverse event (TEAE) of any-grade was differentiation syndrome (12%), followed by neutropenia (12%)
- Grade ≥3 differentiation syndrome was observed in 5% of patients
- Differentiation syndrome was reported as a dose-limiting toxicity (DLT) in ≥2 patients, managed with close monitoring and administration of steroids or hydroxyurea
- DLT was reported in 8% of patients, including QT prolongation in one patient
Pharmacodynamics
Bone marrow blast reduction was observed in 71% of patients, with over half of patients reporting ≥50% blast reduction; and was similar in patients with KMT2Ar or NPM1 mutations.
Efficacy
A total of 33 patients receiving 45–130 mg of JNJ-75276617 twice daily were included in the efficacy subset analysis, with response rates shown in Figure 1.
- The median time to first response was 1.8 months (range, 0.9–3.3 months), and the median duration of response was 6.5 months (range, 1.0–not reached).
- Of the six patients achieving complete remission (CR), three were measurable residual disease status (MRD) negative.
Figure 1. Response rates in patients receiving escalated doses of JNJ-75276617*
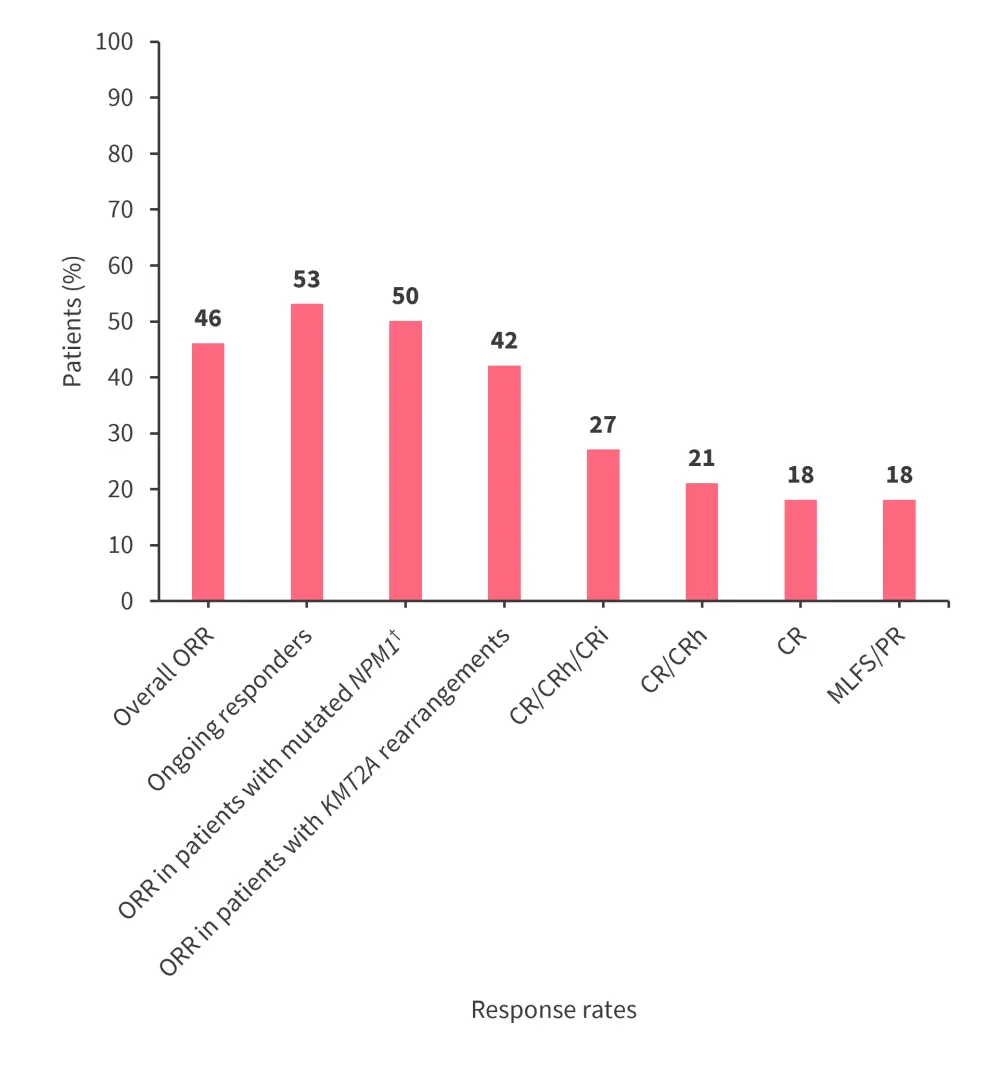
CR, complete remission; CRh, CR with incomplete hematologic recovery; CRi, CR with incomplete recovery; MLFS, morphological leukemia-free state; ORR, overall response rate; PR, partial remission.
*Data from Jabbour.2
†Patients with mutated NPM1 (n = 14) and mutated KMT2A (n = 19).
Revumenib monotherapy in patients with KMT2A-rearranged R/R acute leukemia (AUGMENT-101)3
In this interim analysis from the phase II AUGMENT-101 trial (NCT04065399) of patients with R/R KMT2A-rearranged acute leukemia aged ≥30 days, patients received RP2D of oral revumenib (163 mg every 12 hours) plus a CYP3A4 inhibitor in 28-day cycles.
- The primary endpoint was CR + CR incomplete hematologic recovery (CRh) rate.
- Key secondary endpoints included composite CR, overall response rate, and safety.
Efficacy
At the data cutoff of July 24, 2023, patients in the efficacy analysis (n = 57) had a median age of 34 years (range, 1.3–75 years), 58% of patients were female, and 86% of patients had AML. Patients were heavily pretreated, with 72% having received prior venetoclax and 46% a hematopoietic stem cell transplant (HSCT).
- The primary endpoint was met with 23% of patients achieving CR + CRh (p = 0.0036) (Figure 2).
- Median time to CR + CRh was 1.87 months (range, 0.9–4.6 months), and the median duration of CR + CRh was 6.4 months (95% confidence interval [CI], 3.4–not reached).
- Median OS was 8 months (95% CI, 4.1–10.9 months).
- Overall, 39% of patients proceeded to HSCT, of which 50% continued revumenib posttransplant as maintenance therapy.
Figure 2. Response rates in patients receiving revumenib monotherapy*
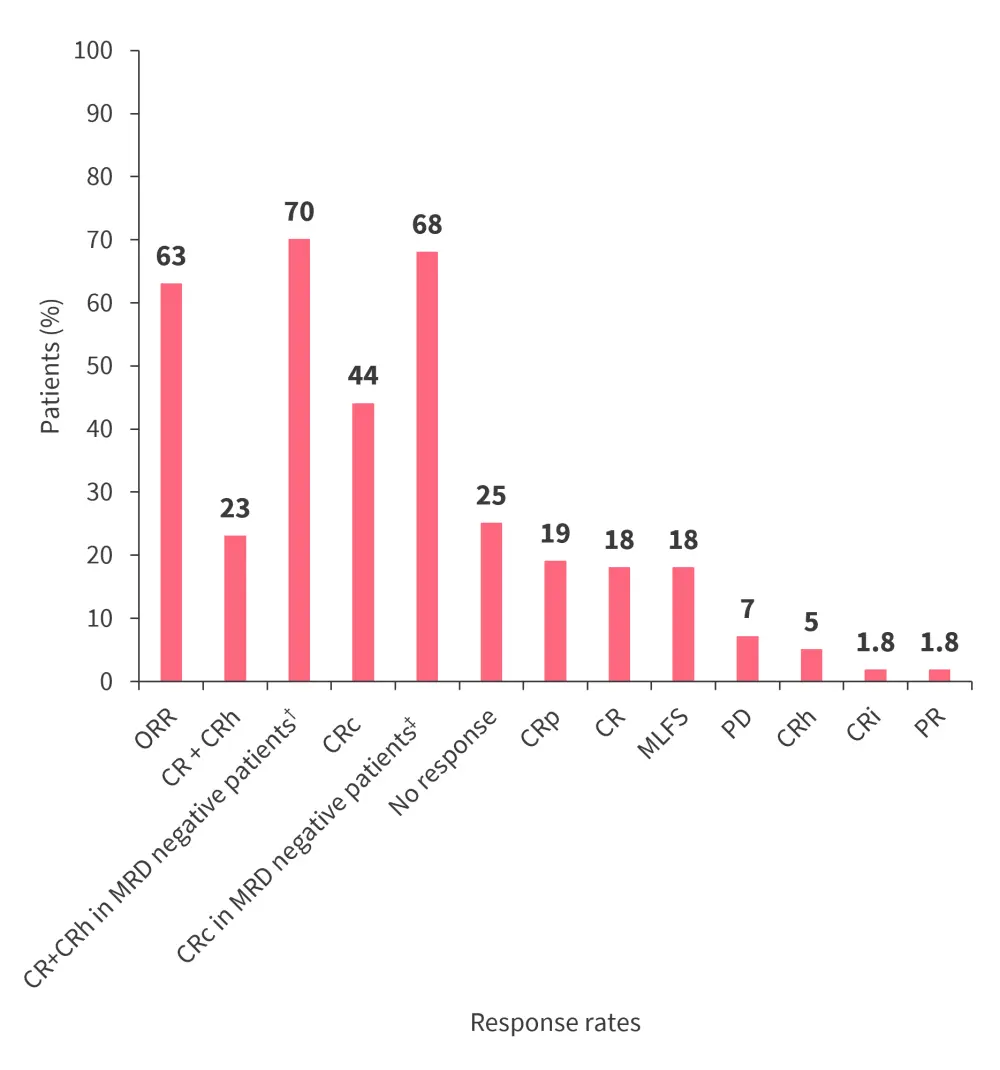
CR, complete remission; CRc, composite CR; CRh, CR with incomplete hematologic recovery; CRi, CR with incomplete recovery; CRp, CR with incomplete platelet recovery; MLFS, morphological leukemia-free state; ORR, overall response rate; PD, progressive disease; PR, partial remission.
*Data from Aldoss.3
†n = 10
‡CRc in MRD negative patients (n = 22).
Safety
Safety analysis (n = 94) found adverse events and TEAE as shown in Figure 3.
- TEAEs leading to dose reduction were reported in 10% of patients, and discontinuation of treatment in 13%.
- No patients discontinued treatment due to differentiation syndrome, corrected QT interval prolongation, or cytopenias.
Figure 3. Most common Grade ≥3 TEAEs occurring in ≥10% of patients*
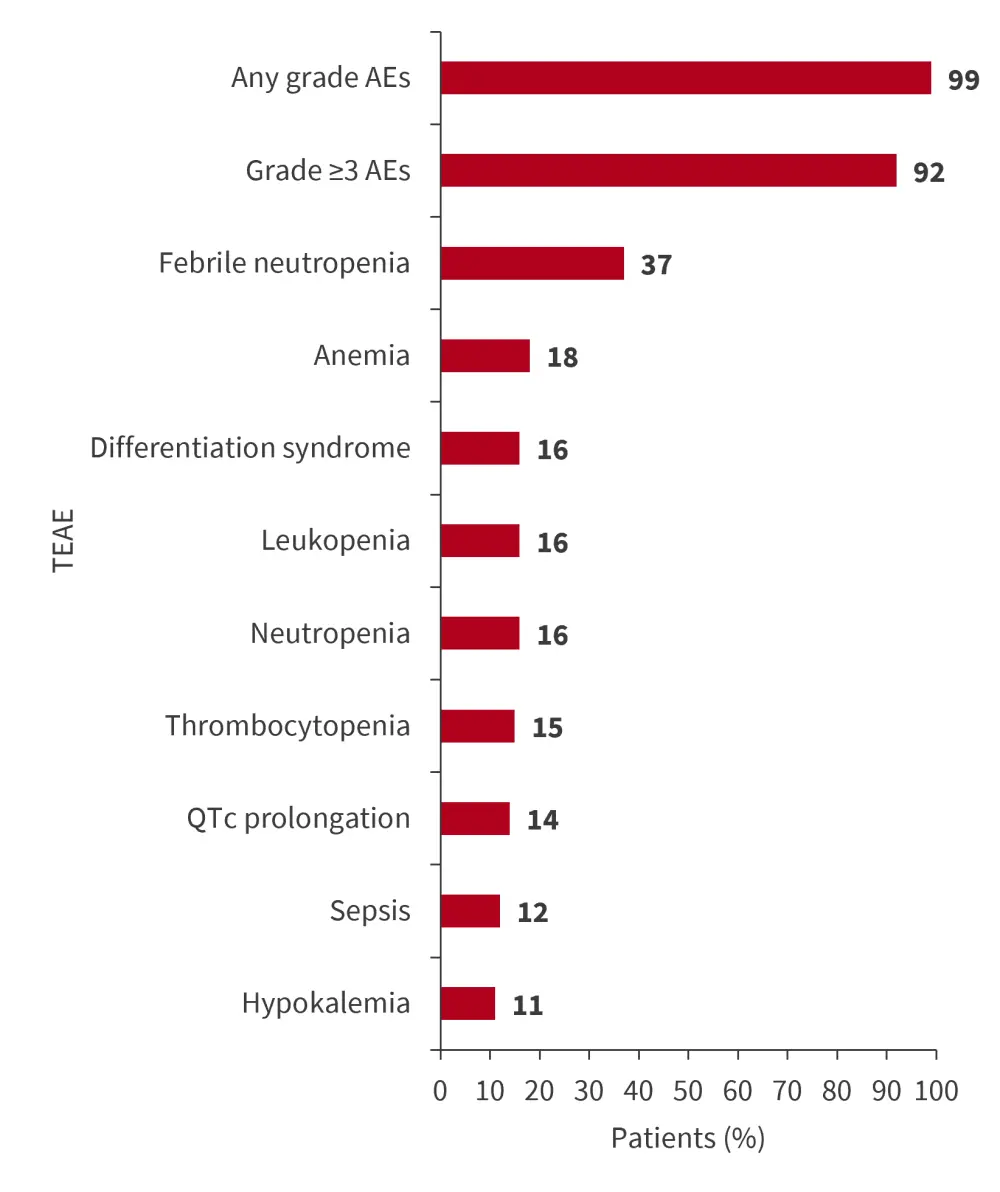
AE, adverse event; TEAE, treatment-emergent adverse event; QTc, corrected QT interval.
*Data from Aldoss.3
Revumenib + decitabine + cedazuridine plus venetoclax (SAVE)4
The SAVE trial (NCT05360160) is a phase I/II dose escalation study in patients aged ≥12 years with R/R AML or mixed phenotype acute leukemia, rearrangements in KMT2A or NUP98 or mutated NPM1, and an Eastern Cooperative Oncology Group score of ≤2.
- The dosing schedule is shown in Figure 4.
- Phase I primary endpoints were safety, tolerability, and RP2D; efficacy was included as a phase II endpoint.
- Phase II secondary endpoints included OS, relapse-free survival, and MRD.
- At the data cutoff of November 1, 2023, nine patients were enrolled, with a median age of 30 years (range, 12–63 years) and three patients aged between 12–18 years.
- Patients were heavily pretreated with a median of 3 prior lines of therapy; 55% had received prior venetoclax and 67% an HSCT.
Figure 4. Dosing schedule*
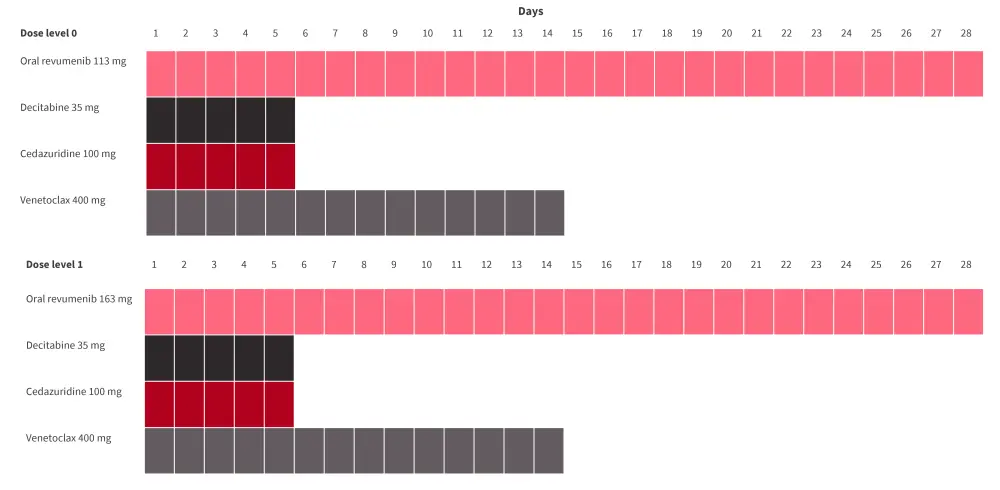
*Data from Issa.4
Safety
- Grade 4 prolonged thrombocytopenia was reported as a DLT at dose levels 0 and 1 but was resolved after a dose hold.
- No patients discontinued treatment due to TEAEs, and the most common TEAEs of any grade were febrile neutropenia, nausea, and hyperphosphatemia (56% each).
- Grade 1–2 differentiation syndrome and QT prolongation occurred in 22% and 33% of patients, respectively.
- Grade ≥3 treatment-related AEs are shown in Figure 5.
Figure 5. Most common Grade ≥3 TRAEs*
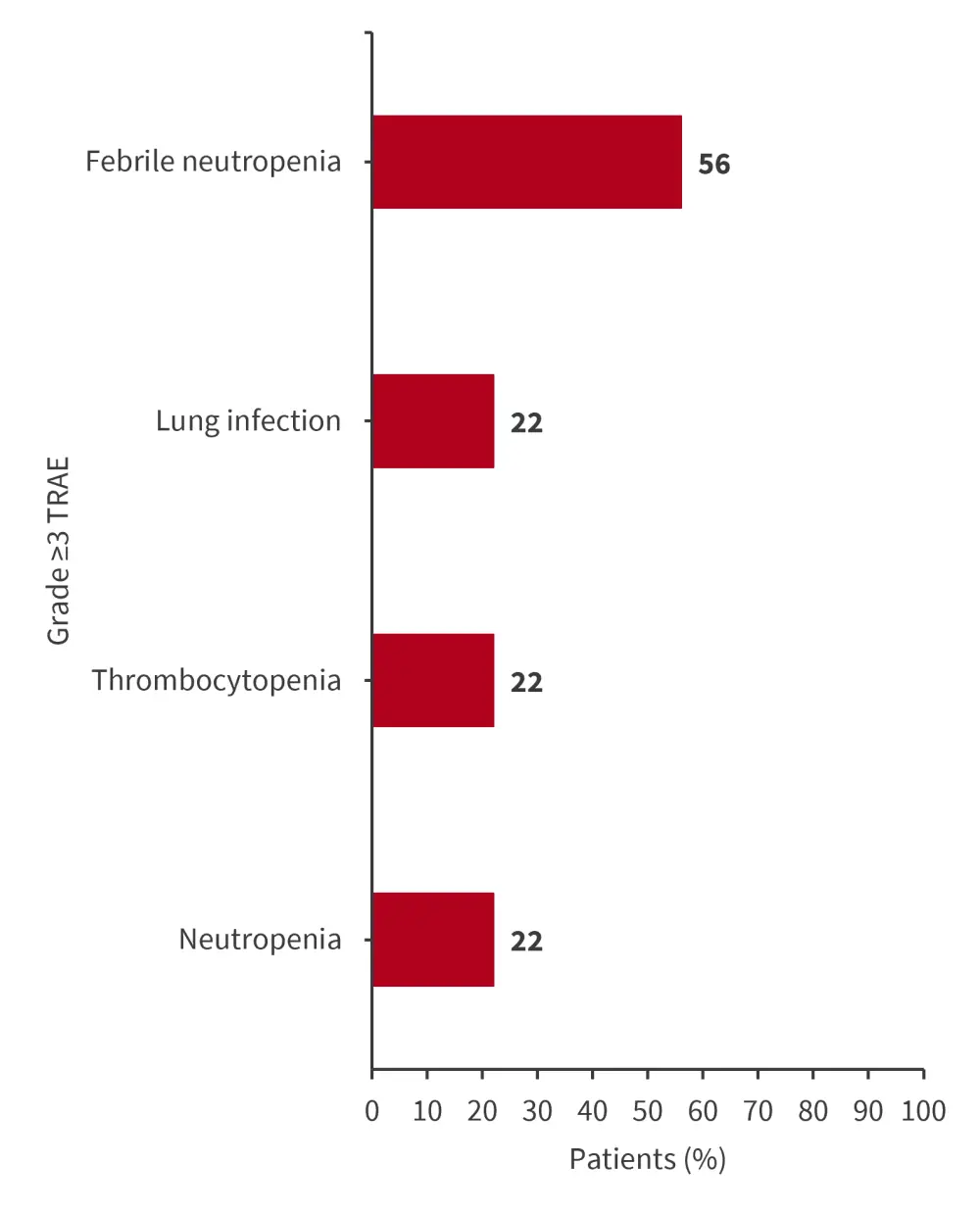
TRAE, treatment-related adverse event.
*Data from Issa.3
Pharmacokinetics
Plasma concentrations of revumenib in the SAVE trial were found to be comparable with revumenib monotherapy (mean concentrations 8.05 vs 7.11).
Efficacy
At a median follow-up of 6.4 months (range, 0.4–12.3 months), revumenib led to rapid responses with an overall response rate of 100% (Table 1). Response rates in the genetic subgroups are shown in Table 1.
- The median time to response was 28 days (range, 14–55 days)
- Bone marrow blasts were reduced to <5% in six patients on Day 14.
- Five patients underwent HSCT consolidation, with two of these receiving revumenib maintenance posttransplant.
Table 1. Response rates in patients receiving revumenib in combination with decitabine + cedazuridine plus venetoclax*
|
Patients, % |
All patients |
Patients with KMT2A rearrangement |
Patients with NUP98 rearrangements |
Patients with NPM1 mutations |
|---|---|---|---|---|
|
ORR |
100 |
100 |
100 |
100 |
|
CR/CRh |
44 |
60 |
33 |
0 |
|
CRp |
33 |
40 |
0 |
100 |
|
PR |
11 |
0 |
33 |
0 |
|
MLFS |
11 |
0 |
33 |
0 |
|
MRD-negativity by MFC |
67 |
80 |
33 |
100 |
|
In CR/CRh |
100 |
100 |
100 |
100 |
|
6-month RFS |
62.2 |
0 |
0 |
0 |
|
6-month OS |
71.1 |
— |
— |
— |
|
CR, complete remission; CRc, composite CR; CRh, CR with incomplete hematologic recovery; CRi, CR with incomplete recovery; CRp, CR with incomplete platelet recovery; MFC, multiparametric flow cytometry; MLFS, morphological leukemia-free state; MRD, measurable residual disease; ORR, overall response rate; OS, overall survival; PR, partial remission; RFS, relapse-free survival. |
||||
Proteomic analysis of revumenib5
Leukemia-focused 51-parameter CyTOF panels were used pre and posttreatment in patients treated with revumenib in a phase I trial (n = 5).
- Proteomic analysis revealed quantitative changes in cell composition and blast reduction with revumenib.
- CD11b+/CD68+ showed enrichment along with differentiated monocytic cells situated near AML blasts, and depletion of immature AML blasts.
- Single cell DNA + protein analysis found enrichment of differentiated AML blasts with monocytic/dendritic cell phenotypes and harboring AML mutations.
- Differentiated AML blasts expressed higher levels of MCL1 and downregulated MEIS1, PBX3, BCL-2, and BCL-xL.
Ziftomenib + selinexor6
This preclinical study assessed the synergistic growth inhibition of ziftomenib in combination with selinexor, a selective inhibitor of nuclear export, on AML cells. The impact on survival was assessed using xenografted mice.
Key findings
- Zifotmenib + selinexor synergistically inhibited the growth of MV-11 and MOLM13 mixed lineage leukemia-rearranged (MLL-r) AML cell lines and suppressed colony formation of CD34+ MLL-r progenitor stem cells.
- Flow cytometry analysis showed that ziftomenib + selinexor induced higher apoptosis and robust reduction of G2/M cell population vs single agent in MV4-11 AML cells.
- Proteomic analysis suggested that CCNB2, TTK, CDKN2A, and CDK4 regulatory proteins are downregulated.
- Ziftomenib (50 mg/kg) + selinexor (5 mg/kg or 2.5 mg/kg) improved survival vs monotherapy of either agent in GDP/Luc+ MV4-11 xenografted mice.
- Survival was also improved in patient-derived xenografted NSG (NOD scid gamma) mice treated with ziftoemnib (50 mg/kg) + selinexor (7.5 mg/kg)
Ziftomenib + standard-of-care therapies7
The phase I KOMET-008 trial (NCT06001788) will evaluate the safety and preliminary efficacy of ziftomenib in combination with fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin (FLAG-Ida), or low-dose cytarabine in patients with R/R NPM1-mutated or KMT2Ar AML. The combination of ziftomenib + gilteritinib will also be investigated in patients with R/R NPM1-mutated AML with an FLT3 comutation.
- This is a 2-part dose-escalation and expansion trial, with six patients treated at dose level 1 within the respective cohorts.
- Dose escalation will occur independently based on an i3 + 3 design to determine ziftomenib doses for the expansion phase.
- The primary endpoint of this trial is the safety and tolerability of the ziftomenib combinations, as assessed by the rate of dose-limiting toxicities per dose level, and adverse events.
Conclusion
These presentations demonstrate the potential of menin inhibitors in the treatment of AML. Revumenib was shown to be safe and effective in both pediatric and adult patients with R/R KMT2A-rearranged AML, demonstrating durable MRD-negative remissions and high transplant rates in responders. The SAVE combination of revumenib with decitabine, cedazuridine, and venetoclax suggests a good response rate and well tolerated safety profile. Furthermore, the proteomic analysis found that revumenib can cause proteomic alterations and changes to the leukemia proteomic landscape including downregulation of MEIS1, PBX3, BCL-2, and BCL-xL.
Also, JNJ-75276617 was found to have a well tolerated safety profile and efficacy in patients with R/R KMT2A-rearranged or NPM1-mutated AML, in an ongoing dose escalation study is ongoing.
Preclinical data suggest that the simultaneous inhibition of the menin-KMT2a interaction and nuclear export may be an effective treatment for patients with MLL-r AML. The KOMET-008 trial will determine the safety and preliminary clinical activity of ziftomenib in combination with standard-of-care therapies in patients with R/R KMT2A-rearranged or NPM1-mutated AML.
Several menin inhibitors in the preliminary stages of clinical development show promising initial results. Further studies are warranted to determine the full therapeutic potential of these treatments in patients with AML.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

