All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Editorial theme │ Navigating the recent updates to the classification of AML: Validation and refinement of the 2022 ELN classification
Do you know... Based on the refined classification proposed by Rausch et al., a patient carrying CBFB::MYH11 or CEBPAb (ZIP-inf) without cytogenetic changes would be classed under which risk group?
The European LeukemiaNet (ELN) released an update to their 2022 recommendations in 2022 following changes in the classification of acute myeloid leukemia (AML), genomic diagnostics, and molecular markers. The AML Hub has previously covered the ELN recommendations, including the updated 2022 ELN classification.
During the European Hematology Association (EHA) 2023 Congress, Bill.1 presented validation of the updated 2022 ELN risk stratification in adult patients with AML. In addition, Rausch et al.2 recently published a study on the validation and refinement of the 2022 ELN genetic risk stratification of AML. As part of our editorial theme on navigating the recent updates to the classification of AML, we are pleased to summarize their key findings.
Improved prognostic value with the ELN 2022 vs ELN 2017 classification1
A large cohort study in adult patients with newly diagnosed AML treated within the German Study Alliance Leukemia trials. The median age of included patients was 54 years (range, 18–89 years).
Results
At a median follow-up of 6.9 years, 1,570 patients were classified according to the 2022 ELN classification. Comparison of pretreatment parameters based on the ELN groups revealed significant differences (Table 1).
Table 1. Pretreatment parameters based on 2022 ELN groups*
|
ELN, European LeukemiaNet; sAML, secondary acute myeloid leukemia; tAML, therapy-related acute myeloid leukemia; WBC, white blood cell. |
||||
|
Pretreatment parameters, % (unless stated otherwise) |
Favorable-risk |
Intermediate-risk |
Adverse-risk |
p value |
|---|---|---|---|---|
|
Median age (range), years |
51 (18–85) |
55 (18–85) |
56 (18–89) |
<0.001 |
|
Diagnosis |
|
|
|
<0.001 |
|
de novo AML |
94.2 |
86.5 |
72.3 |
|
|
sAML |
3.8 |
10.6 |
22.3 |
|
|
tAML |
1.9 |
3.0 |
5.4 |
|
|
Median WBC count (range), 109/L |
27.5 (0.3–453) |
23.4 (0.6–466) |
9.2 (0.4–450) |
<0.001 |
|
Median blood blasts (range) |
45 (0–98) |
45 (0–100) |
28 (0–99) |
<0.001 |
|
Median bone marrow blasts (range) |
64 (7–96) |
68 (20–100) |
56 (20–100) |
<0.001 |
Survival outcomes
Differences between the 2022 ELN groups in complete remission (CR), disease-free survival, and overall survival (OS) are shown in Table 2. Although 21% (n = 340) of patients were reclassified using 2022 ELN recommendations compared with 2017 ELN recommendations, the net effect on the percentage of patients reclassified was minimal (favorable-risk group, −0.7%; intermediate-risk group, −3.4%; adverse-risk group, +4.1%). The movement to each reclassified risk group was due to:
- favorable-risk, bZIP in-frame mutated CEBPA
- intermediate-risk, loss of the FLT3-ITD allelic ratio
- adverse-risk, secondary type mutations
Patients reclassified according to the 2022 vs 2017 ELN recommendations showed an improved 3-year OS (p = 0.001) and allowed better separation of the risk groups.
- Patients in the 2022 ELN favorable-risk group with CBF-AML and CEBPA bZIP mutations vs NPM1 mutations showed superior OS.
- Patients in the 2022 ELN adverse-risk group with TP53 mutations had the worst OS, followed by those with ASXL1/RUNX1 mutations and those with secondary type mutations.
Table 2. Survival outcomes based on 2022 ELN groups*
|
CR, complete remission; DFS, disease-free survival; ELN, European LeukemiaNet; OS, overall survival. |
||||
|
Survival outcomes, % (unless stated otherwise) |
Favorable-risk |
Intermediate-risk |
Adverse-risk |
p value |
|---|---|---|---|---|
|
CR |
87.3 |
76.6 |
49.2 |
<0.001 |
|
5-year OS |
53 |
32 |
13 |
<0.001 |
|
5-year DFS |
49† |
32‡ |
23§ |
<0.001 |
ELN 2022 classification identifies more patients in the adverse-risk group2
In a cohort study of patients with newly diagnosed AML treated with cytarabine-based induction chemotherapy in two German AML Cooperative Group trials, the median age was 58 years (range, 18–86 years). The validation cohort included 1,160 patients with AML, of which 83% were aged <60 years and received intensive induction chemotherapy.
Results
A total of 1,118 patients were classed according to the 2022 ELN classification (Figure 1).
Figure 1. 2022 ELN categories stratified by age and sex*
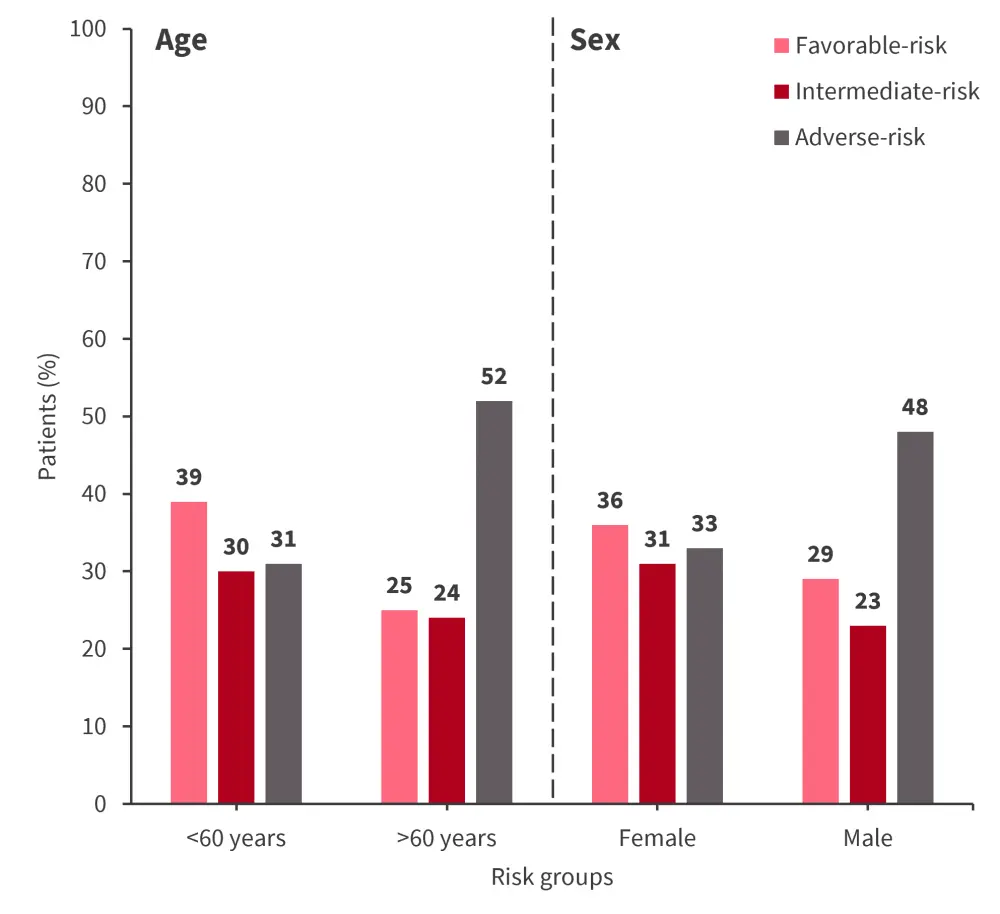
ELN, European LeukemiaNet.
*Adapted from Rausch, et al.2
- The 2022 ELN adverse-risk group was associated with older age (p < 0.0001), male sex (p = 0.003), secondary AML (p = 0.0006), and a lower white blood cell (WBC) count at diagnosis (p < 0.0001) vs ELN favorable/intermediate-risk.
- The higher proportion of male patients was due to a lower prevalence of NPM1 mutations, and a higher prevalence of RUNX1 and ASXL1 mutations (p < 0.0001 and p = 0.009, respectively).
- Male sex was also associated with the adverse-risk-defining mutations EZH2 (p = 0.0002), SRSF2 (p < 0.0001), STAG2 (p = 0.0075), U2AF1 (p = 0.0016), and ZRSR2 (p = 0.0005).
- In total, 85% of patients remained in the same risk group in both the 2022 and 2017 ELN classifications (weighted kappa, 0.84; 95% confidence interval [CI], 0.87–0.89).
- Of the 15% reclassified patients, 3% moved into a more favorable-risk group and 12% into a less favorable-risk group.
Figure 2 shows response and survival rates for patients classified by 2022 ELN risk groups.
Figure 2. CR, RFS, and OS rates for 2022 ELN risk groups*
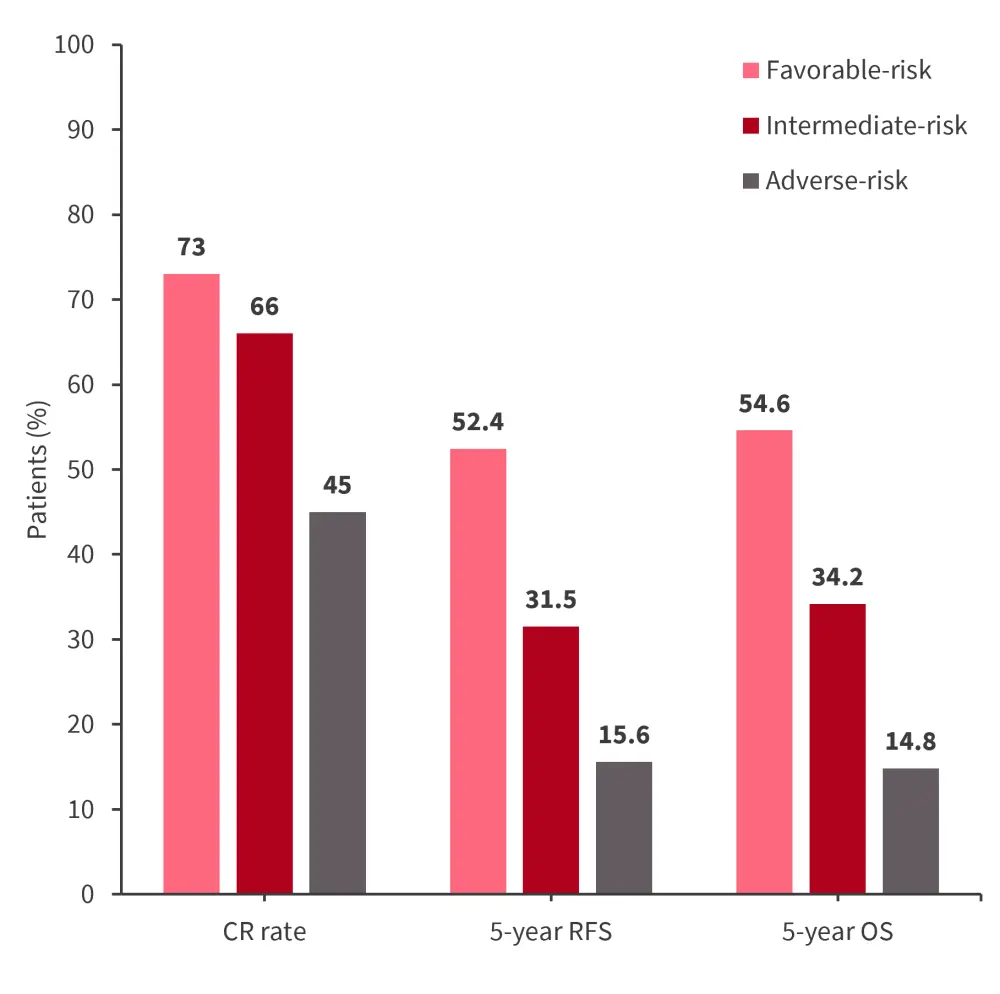
CR, complete remission; ELN, European LeukemiaNet; OS, overall survival; RFS, relapse-free survival.
*Adapted from Rausch, et al.2
- Multivariate analysis found older age (p = 0.003), higher WBC count (p = 0.04), and a diagnosis of secondary (p = 0.002) or therapy-related AML (p = 0.0004) were associated with lower likelihood of achieving CR in the 2022 ELN adverse-risk group.
- Older age (p = 0.008 and p < 0.0001) and higher WBC count (p = 0.006 and p = 0.003) were associated with shorter relapse-free survival (RFS) and OS, respectively.
- The prognostic accuracy of 2022 ELN for OS was lower compared with 2017 ELN (Harrel’s C-index, 0.658 vs 0.664, respectively).
Improved OS in patients reclassified from the ELN 2017 intermediate- to ELN 2022 adverse-risk group
Patients reclassified from the 2017 ELN intermediate- to 2022 ELN adverse-risk group demonstrated improved 5-year OS (Figure 3). Patients reclassified from the 2017 ELN adverse- to the 2022 ELN intermediate-risk group had numerically worse 5-year OS. The OS was also significantly worse compared with those reclassified from the favorable- to intermediate-risk group (p = 0.016).
Figure 3. OS in patients reclassified from ELN 2017 to ELN 2022*
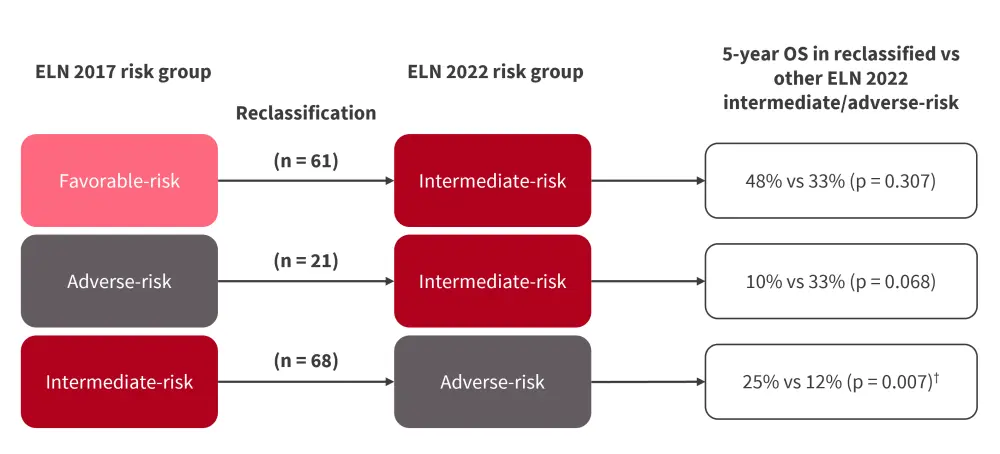
ELN, European LeukemiaNet; OS, overall survival.
*Adapted from Rausch, et al.2
†Statistically significant.
Outcomes stratified by post-remission therapy and genetics
Allogeneic hematopoietic stem cell transplantation in the first CR was associated with 2022 ELN risk groups (18% of favorable-, 30% of intermediate-, and 34% of adverse-risk patients; p < 0.0001). Patients in the adverse-risk group receiving allogeneic hematopoietic stem cell transplantation in the CR had improved OS (p = 0.032). In addition, OS was superior in patients in intermediate- and adverse-risk groups with low FLT3- internal tandem duplication (ITD) compared with high FLT3-ITD (45% vs 27%; p = 0.097 and 25% vs 10%; p = 0.027, respectively).
Based on the presence of myelodysplasia-related (MR) mutations, 45% of reclassified patients moved from the 2017 ELN favorable- and intermediate-risk groups to the 2022 ELN adverse-risk group. 5-year RFS (p = 0.0035) and OS (p = 0.0004) were improved in patients with MR mutations vs other adverse-risk mutations. The validation cohort confirmed improved 5-year OS for patients reclassified to the adverse-risk group with MR mutations compared with other adverse-risk patients (30% vs 18%, p = 0.0052). However, OS was significantly worse in reclassified patients with MR mutations than the remaining intermediate-risk group (p = 0.02).
Refinement of 2022 ELN risk classification
The authors proposed refinement of the 2022 ELN risk classification system to include very favorable- and very adverse-risk groups, without any additional genetic markers.
- This revealed that patients with CBFB::MYH11 or CEBPAbZIP-inf mutations (very favorable-risk group) had superior OS (71% and 60%), compared with those with RUNX1::RUNX1T1 or NPM1mut without FLT3mut (OS, 50% and 51%).
- Patients with complex karyotype in combination with TP53 mutations (very adverse-risk group) had worse outcomes (RFS and OS, 0% for both).
- Figure 4 shows validation of the proposed refined ELN 2022 classification in the AML Study Group cohort for response and survival rates.
- The validation cohort confirmed a trend towards improved 5-year OS in the very favorable- vs favorable-risk group (77% vs 58%, p = 0.06), and worse 5-yr OS in the very adverse-risk group vs adverse-risk group (0% vs 24%; p < 0.001).
- Multivariate analyses showed that the very adverse-risk group had the poorest CR, RFS, and OS compared with the adverse-risk group, whereas the very favorable-risk group demonstrated an OS benefit compared with the favorable-risk group.
Figure 4. Validation of proposed refinement of 2022 ELN classification for CR and OS rates *
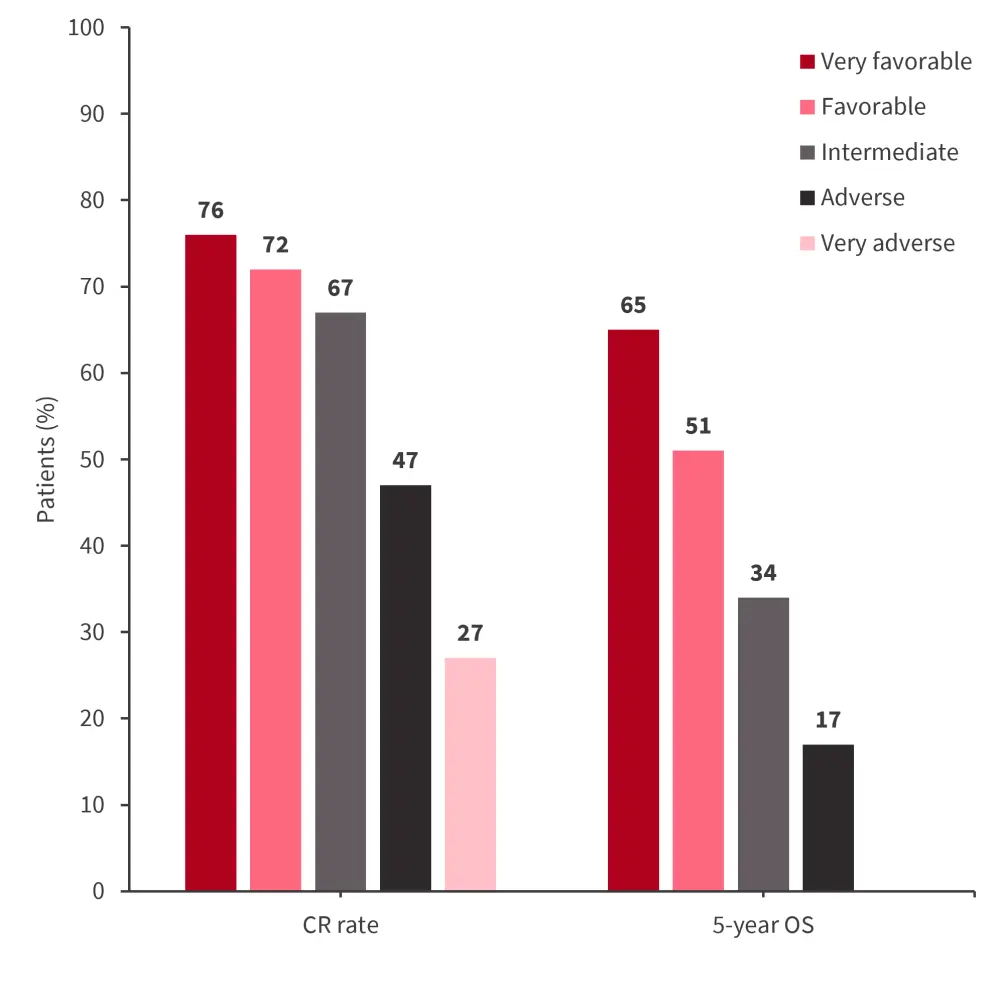
CR, complete remission; ELN, European LeukemiaNet; OS, overall survival.
*Adapted from Rausch, et al.2
Conclusion
The study found the 2022 ELN risk stratification more predictive than the ELN 2017 risk stratification, with minimal impact despite a 21% reclassification rate. Additional analysis on secondary mutations in the adverse-risk group is needed.
The study by Rauch et al.2 showed the 2022 ELN classification assigned more patients to an adverse-risk group, yielding better outcomes than the 2017 ELN adverse-risk group. The inclusion of MR mutations outlines the need to consider them in the intermediate-risk group. Further refinement of the 2022 ELN classification is warranted to address the unmet needs of patients with a very poor prognosis.
Your opinion matters
The updated ELN 2022 guidelines has introduced a protocol for diagnosing AML in cases where blast cell count is equal to or greater than 10%, using recurring genetic abnormalities. Does this increase the need for broad molecular profiling upfront and early for patients with AML?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




