All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Efficacy and safety of FLT3 inhibitors in the treatment of AML: A review of clinical data
Do you know... Which FLT3 inhibitor has FDA approval in induction, consolidation, and post-consolidation maintenance treatment regimens for newly diagnosed FLT3-ITD AML and is also under investigation in patients who are FLT3-ITD-negative?
FMS-like tyrosine kinase 3 (FLT3) mutations are present in ~30% of patients with acute myeloid leukemia (AML).1 The most common type of mutation is internal tandem duplication (ITD), representing ~25% of FLT3-mutated (FLT3m) cases, while mutations in the tyrosine kinase domain (TKD) occur in ~7–10% of cases.1 FLT3 mutations are strong drivers of leukemogenesis and are key therapeutic targets in FLT3m AML.2 In the last few years, several FLT3 inhibitors have been developed with variable targeting properties, pharmacokinetics , efficacy, and toxicity profiles. Based on their binding ability, FLT3 inhibitors are classified into Type I, which bind to the active conformation of the receptor and target both the ITD and TKD-mutated FLT3 receptor, or Type II, which bind only to the inactive or the ITD conformation and are not active against TKD mutations (Figure 1).2
Figure 1. Mechanism of action of Type I and II FLT3 inhibitors*
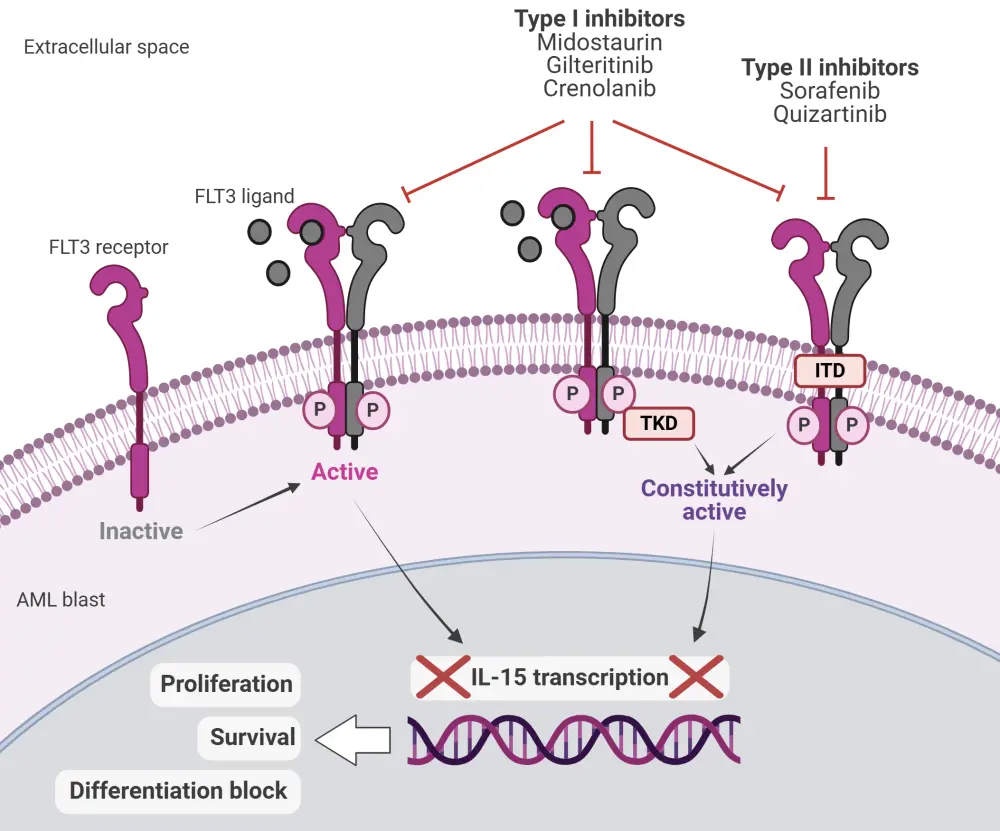
AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase; IL, interleukin; ITD, internal tandem duplication; P, phosphate; TKD, tyrosine kinase domain.
*Adapted from Biavasco and Zeiser2 in accordance with the Creative Commons Attribution Non-Commercial License (CC BY-NC 4.0). Created with Biorender.com.
Below, we summarize data from key clinical trials evaluating the efficacy and safety of FLT3 inhibitors in the treatment of AML.
Type I FLT3 inhibitors
Midostaurin
Midostaurin was the first FLT3 inhibitor approved by the U.S. Food and Drug Administration (FDA) and the European Commission (EC) in combination with standard chemotherapy for the treatment of patients with newly diagnosed (ND) FLT3m AML. The approval was based on results from the phase III RATIFY trial (Table 1).1,3
Table 1. Key trials of midostaurin in AML*
|
Regimen |
Trial |
Patient population |
Key efficacy outcomes |
Key safety outcomes |
|
Induction with midostaurin + standard chemotherapy vs placebo, followed by consolidation with HDAC, followed by maintenance with midostaurin monotherapy3 |
Phase III RATIFY (NCT00651261) |
717 ND patients with FLT3-ITD and -TKDm AML |
Induction therapy |
Grade ≥3 anemia: 92.7% vs 87.8% (p = 0.03) Grade ≥3 rash: 14.1% vs 7.6% (p = 0.008) Nausea: 5.6% vs 9.6% (p = 0.05) |
|
AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase 3; HDAC, high-dose cytarabine; ITD, internal tandem duplication; mOS, median overall survival; ND, newly diagnosed; TKDm, tyrosine kinase domain-mutated. |
||||
Read about the 10-year follow-up data of the RATIFY trial, presented during the 66th American Society of Hematology (ASH) Annual Meeting and Exposition, here.
Gilteritinib
Gilteritinib is approved by the U.S. FDA and the EC for the treatment of patients with FLT3m relapsed/refractory (R/R) AML, based on results of the phase III ADMIRAL trial (Table 2).4 The findings were further validated by interim results from the ongoing COMMODORE trial, which predominantly involved Asian patients with R/R FLT3m AML.5 Gilteritinib was also evaluated as post- hematopoietic stem cell transplantation (HSCT) maintenance treatment in patients with FLT3m R/R AML in the phase III MORPHO trial.6 The primary endpoint of relapse-free survival (RFS) was not met; however, RFS was higher in patients with detectable FLT3-ITD measurable residual disease (MRD) pre- or post-HSCT who received gilteritinib treatment.6 A post-hoc analysis of the MORPHO trial found that assessing FLT3-ITD MRD may help determine which patients could benefit from gilteritinib maintenance.7 The phase III LACEWING trial evaluating gilteritinib + azacitidine vs azacitidine alone in ND patients with FLT3m AML ineligible for intensive chemotherapy did not meet its primary endpoint and the study was closed based on protocol-specified boundary for futility.8
Table 2. Key trials of gilteritinib in AML*
|
Regimen |
Trial |
Patient population |
Primary endpoints |
Safety profile |
|
Gilteritinib monotherapy (120 mg/day) vs salvage chemotherapy9 |
Phase III ADMIRAL (NCT02421939) |
371 patients with FLT3-ITD or FLT3-TKDm R/R AML |
mOS: 9.3 months vs 5.6 months (p < 0.001) mEFS: 2.8 months vs 0.7 months CR/CRh: 34% vs 15.3% |
Most common Grade ≥3 AEs with gilteritinib: febrile neutropenia (45.9%), anemia (40.7%), thrombocytopenia (22.8%) |
|
Gilteritinib monotherapy (120 mg/day) vs salvage chemotherapy5 |
Phase III COMMODORE (NCT03182244; ongoing) |
234 Asian patients with FLT3m R/R AML† |
mOS: 9.6 months vs 5.0 months (p = 0.0021) mEFS: 2.8 months vs 0.6 months (p = 0.00004) |
Exposure-adjusted Grade ≥3 AEs: |
|
Gilteritinib vs placebo as post-HSCT maintenance6 |
Phase III MORPHO (NCT02997202) |
356 patients with FLT3-ITD AML |
2-year RFS: 77.2% vs 69.9% (p = 0.0518) |
Grade ≥3 TEAE: 82% vs 53.1% |
|
Gilteritinib + azacitidine vs azacitidine alone8 |
Phase III LACEWING (NCT02752035) |
123 patients with ND FLT3m AML ineligible for IC |
mOS: 9.82 months vs 8.87 months (p = 0.753) |
Grade ≥3 AEs: 95.9% vs 89.4% Most common AEs with gilteritinib + azacitidine: pyrexia (47.9%), diarrhea (38.4%) |
|
AE, adverse event; AML, acute myeloid leukemia; CR, complete remission; CRh, CR with partial hematologic recovery; E/PY, event/patient-year; HSCT, hematopoietic stem cell transplantation; IC, intensive chemotherapy; m, median; OS, overall survival; RFS, relapse-free survival; R/R, relapsed/refractory; TKDm, tyrosine kinase domain-mutated. |
||||
Learn about the timing of response with gilteritinib through a post hoc analysis of the ADMIRAL and COMMODORE trials, presented at the 66th ASH Annual Meeting and Exposition, here.
A phase III trial (HOVON 156 AML; NCT04027309) of gilteritinib vs midostaurin in combination with induction and consolidation treatment for FLT3m AML is currently ongoing.4
Crenolanib
Crenolanib is an investigational FLT3 inhibitor that received FDA fast track designation for the treatment of patients with R/R FLT3m AML. A phase II trial of crenolanib in combination with intensive chemotherapy in ND patients with FLT3m AML showed deep responses, long-term survival, and an acceptable safety profile (Table 3).9 Results from two phase III trials; NCT03258931 (comparing crenolanib and midostaurin in combination with cytarabine and daunorubicin) and NCT03250338 (crenolanib in combination with chemotherapy vs chemotherapy alone) are awaited.
Table 3. Key trials of crenolanib in AML*
|
Regimen |
Trial |
Patient population |
Key efficacy outcomes |
Safety profile |
|
Crenolanib + IC10 |
Phase II (NCT02283177) |
44 patients with ND FLT3-ITD and -TKDm AML |
ORR: 86% CR: 77% (MRD negativity: 89%) CRi: 9% (MRD negativity: 45%) mOS: NR† mEFS: 44.7 months† |
Most common TEAEs: diarrhea (65.9%), nausea (56.8%), febrile neutropenia (52.3%), vomiting (45.5%), peripheral edema (40.9%) |
|
AML, acute myeloid leukemia; CR, complete remission; CRi, CR with incomplete count recovery; EFS, event-free survival; IC, intensive chemotherapy; ITD, internal tandem duplication; m, median; MRD, measurable residual disease; ND, newly diagnosed; ORR, overall response rate; OS, overall survival; TEAE, treatment-emergent adverse event; TKDm, tyrosine kinase domain-mutated. |
||||
Type II FLT3 inhibitors
Sorafenib
Sorafenib combined with chemotherapy was evaluated in the SORAML trial in patients with ND FLT3m AML; the results did not support its use in intensive first-line treatment, but they highlighted its potential as a maintenance therapy after allo-HSCT or in combination with azacitidine or cytarabine in R/R settings.11 The benefit of sorafenib as post-HSCT maintenance was confirmed in the phase II SORMAIN trial and a phase III trial (NCT02474290).12,13
Table 4. Key trials of sorafenib in AML*
|
Regimen |
Trial |
Patient population |
Primary endpoint(s) |
Safety profile |
|
Sorafenib + chemotherapy and as post-HSCT maintenance11 |
Phase II SORAML (NCT00893373) |
267 fit patients aged ≤60 years with ND AML |
5-year EFS: 41% vs 27% (p = 0.011) 5-year RFS: 53% vs 36% (p = 0.035) 5-year OS: 61% vs 53% (p = 0.282) |
No new safety signals |
|
Sorafenib vs placebo as post-HSCT maintenance12 |
Phase II SORMAIN (DRKS00000591) |
83 patients with FLT3-ITD-AML |
24-month RFS: 53.3% vs 85.0% (p = 0.002) |
Most common Grade ≥3 AEs: aGvHD/cGvHD (76.8% vs 59.8%), GI toxicity (14.3% vs 15.4%), infections (26.2% vs 23.1%) |
|
Sorafenib as post-HSCT maintenance13 |
Phase III (NCT02474290) |
202 patients with FLT3-ITD AML |
OS: 72.0% vs 55.9% (p = 0.011) LFS: 70.0% vs 49.0% (p = 0.0007) |
No treatment-related deaths cGvHD 5-year incidence similar between groups (54.0% vs 51.0%; p = 0.73) |
|
AE, adverse event; aGvHD, acute graft-versus-host disease; AML, acute myeloid leukemia; cGvHD, chronic GvHD; EFS, event-free survival; HSCT, hematopoietic stem cell transplantation; LFS, leukemia-free survival; ND, newly diagnosed; OS, overall survival; RFS, relapse-free survival. |
||||
Quizartinib
Quizartinib monotherapy showed improved survival vs salvage chemotherapy and a manageable safety profile in patients with R/R FLT3-ITD AML in the phase III QuANTUM-R trial (Table 5),14 leading to its approval in Japan in this setting.15 It is also approved by the FDA, EC, and in Japan in combination with standard cytarabine and anthracycline induction, cytarabine consolidation, and as a maintenance monotherapy following consolidation chemotherapy for the treatment of patients with ND FLT3-ITD AML. These approvals were based on the positive results from the QuANTUM-First trial.16 Results from the phase III PETHERMA QUIWI trial of quizartinib in combination with standard chemotherapy in patients with ND FLT3-ITD-negative AML were presented at the 66th ASH Annual Meeting and Exposition.17,18 The phase III trial QuANTUM-Wild trial of quizartinib in combination with standard intensive induction and consolidation followed by single-agent maintenance in ND FLT3-ITD-negative AML is in progress.19
Table 5. Key trials of quizartinib in AML*
|
Regimen |
Trial |
Patient population |
Key efficacy outcomes |
Safety profile |
|
Quizartinib monotherapy vs salvage chemotherapy14 |
Phase III QuANTUM-R (NCT02039726) |
367 patients with R/R FLT3-ITD AML |
mOS: 6.2 months vs 4.7 months (p = 0.02) |
Most common non-hematologic Grade ≥3 TEAEs: |
|
Quizartinib vs placebo + standard chemotherapy induction and consolidation, followed by continuation monotherapy16 |
Phase III QuANTUM-First (NCT02668653)
|
539 patients with ND FLT3-ITD AML |
mOS: 31.9 months vs 15.1 months (p = 0.032) |
Most common Grade 3/4 hematologic AEs: Most common Grade 3/4 non-hematologic AEs: |
|
Phase II QUIWI (NCT04107727) |
273 patients with FLT3-ITD-negative AML |
mEFS: 18.8 months vs 9.9 months (p = 0.045) mOS: NR vs 29.3 months (p = 0.01) CR/CRi: 77.2% vs 76.3% |
Most common (≥20%) Grade ≥3 AEs: |
|
|
AE, adverse event; AML, acute myeloid leukemia; CR, complete remission; CRi, CR with incomplete count recovery; EFS, event-free survival; ITD, internal tandem duplication; m, median; ND, newly diagnosed; OS, overall survival; R/R, relapsed/refractory; TEAE, treatment-emergent AE. |
||||
Learn about exploratory analyses of the QuANTUM-First trial evaluating quizartinib maintenance in patients with ND FLT3-ITD AML and the impact of baseline gene mutations on quizartinib survival benefits, as presented during 66th ASH Annual Meeting and Exposition.
Conclusion
Over the years, several FLT3 inhibitors have shown promising results in clinical trials. At present, three FLT3 inhibitors—midostaurin, gilteritinib, and quizartinib—are approved for the treatment of ND and R/R patients across different treatment phases, including induction, consolidation, and maintenance. Studies are ongoing to evaluate quizartinib's efficacy in FLT3-ITD-negative AML. More research is needed to explore various combination strategies, identify strategies to overcome resistance, and further explore the benefits of FLT3 inhibitors in maintenance therapy.
This educational resource is independently supported by Daiichi Sankyo. All content was developed by SES in collaboration with an expert steering committee; funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



