All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Maintenance therapy in AML: Latest updates from ASH 2023
Relapse following complete remission (CR) is a significant cause of mortality in patients with acute myeloid leukemia (AML).1 Maintaining remission following chemotherapy remains challenging, and novel strategies to prevent relapse are needed.1,2 Similarly, relapse following allogeneic hematopoietic stem cell transplantation (allo-HSCT) is associated with poor outcomes.3 Maintenance therapies for patients who undergo allo-HSCT can be limited by toxicity towards normal engrafted cells.3
During the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, several presentations discussed maintenance therapy in patients with AML. Hellstrand.1 and van de Loosdrecht.2 discussed immunotherapy-based maintenance strategies following chemotherapy, and DiPersio.3 highlighted maintenance therapy in the posttransplant setting.
Histamine dihydrochloride and low-dose interleukin-2 maintenance therapy1
In this phase III trial, 320 patients with AML in remission following chemotherapy and ineligible for allo-HSCT were randomized to receive either histamine dihydrochloride and low-dose interleukin-2 (HDC/IL-2) maintenance therapy or no treatment. Patients in the treatment arm received HDC/IL-2 subcutaneously twice daily for 10 consecutive 21-day cycles over 18 months, with 3 weeks rest in the first three cycles and 6 weeks rest in the last seven cycles; median follow-up was 48 months. Hellstrand.1 presented a post-hoc analysis from this trial, assessing the efficacy of HDC/IL-2 in two subgroups,
- chemo-responsive patients, defined as patients in first CR after first induction chemotherapy (n = 130); and
- patients with normal karyotype AML, defined as no somatic chromosomal aberrations (n = 72).
Key findings
In the primary analysis:
- HDC/IL-2 was associated with a leukemia-free survival (LFS) benefit vs no treatment in all patients (hazard ratio [HR], 0.76; p = 0.008) and in patients aged <60 years (HR, 0.56; p = 0.005), and an overall survival (OS) benefit in patients aged <60 years (HR, 0.65; p = 0.07)
- The LFS and OS benefit was limited in patients aged >60 years
In the post-hoc analysis:
- HDC/IL-2 was associated with an OS and LFS benefit vs no treatment in both chemo-responsive patients aged <60 years (LFS HR, 0.48; p = 0.001; OS HR, 0.53; p = 0.02) and patients with normal karyotype AML aged <60 years (LFS HR, 0.40; p = 0.006; OS HR, 0.43; p = 0.04)
- The survival benefit was driven by an LFS benefit in patients aged 40–60 years in both the chemo-responsive group (p = 0.002) and the normal karyotype group (p = 0.0004)
Presenter’s conclusion
Based on these results, HDC/IL-2 is selectively effective at preventing relapse in patients with chemo-responsive AML, normal karyotype AML, and those aged 40–60 years.
Vididencel2
The phase II ADVANCE-II trial (NCT03697707) included 20 patients with AML who were in CR but were measurable residual disease (MRD) positive and ineligible for allo-HSCT. Patients received four biweekly doses of vididencel, an allogenic leukemia-derived dendritic cell vaccine, and two booster doses at Weeks 14 and 18. Patients were assessed for MRD at baseline and Weeks 14, 20, and 32. The primary endpoint was MRD response, and secondary endpoints included relapse-free survival (RFS), OS, immunological responses, and safety. Median follow-up was 31.6 months.
Key findings
Overall, 35% of patients had an MRD response (Figure 1).
Figure 1. MRD Response Rate in the ADVANCE-II trial*
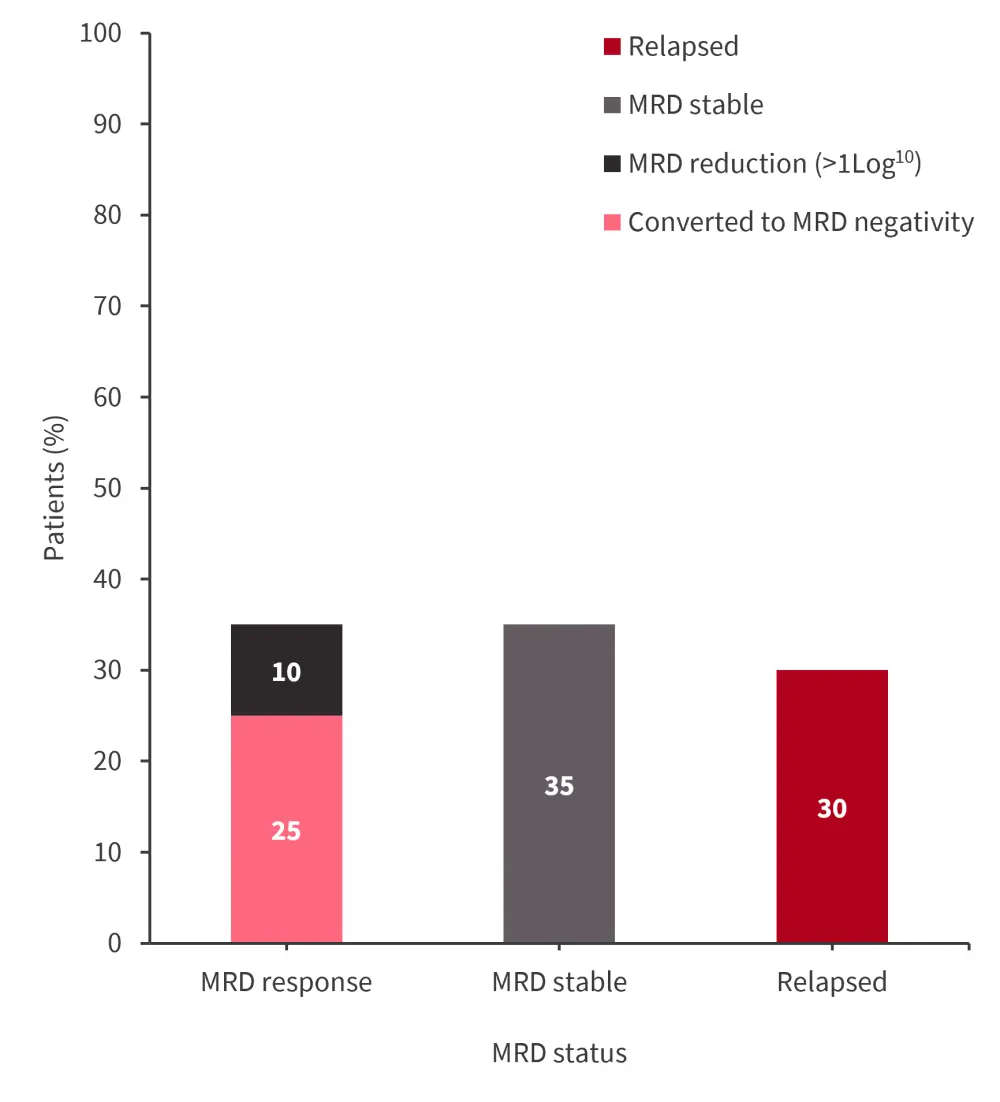
MRD, measurable residual disease.
*Data from van de Loosdrecht.2
MRD, measurable residual disease.
*Data from van de Loosdrecht.2
- Median RFS in all patients was 30.4 months, with an estimated 2-year RFS rate of 56%
- MRD response was associated with an improved RFS vs no MRD response (p = 0.06)
- Median OS was not reached, with an estimated 2-year and 3-year OS of 74.9% and 64.7%, respectively
- OS was longer in patients with an MRD response compared with patients with no MRD response (p = 0.02)
- Tumor antigen-specific T-cell responses were observed in 85% of patients
- The number of vaccine-induced responses was correlated with patients remaining in CR, OS, and MRD response (Table 1)
Table 1. Number of VIR and MRD responses in the ADVANCE-II trial*
|
Response, n |
No VIR |
1–2 VIR |
≥3 VIR |
|---|---|---|---|
|
MRD response |
0 |
2 |
5 |
|
Conversion to MRD negativity |
0 |
1 |
4 |
|
MRD, measurable residual disease; VIR, vaccine-induced response. |
|||
- Imaging mass cytometry showed clear interactions between T cells (CD4 and CD8) and dendritic cells (cDC1 and cDC2) at injection site
- The frequency of dendritic cells after the last dose correlated with OS
- The highest level of dendritic cells was observed in patients with an MRD response
Presenter’s conclusion
Vididencel vaccination in MRD positive patients in CR resulted in a long-term survival benefit, and MRD response was correlated with survival outcomes. Further studies combining vididencel with other maintenance therapies are warranted.
Oral-azacitidine
As covered in our article on updates on maintenance therapy with oral azacitidine from the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, results from several recent analyses support the use of oral azacitidine maintenance therapy after intensive chemotherapy in adult patients with newly diagnosed AML.
Posttransplant maintenance therapy3
The phase I/II VBP101 trial (NCT04849910) assessed tremtelectogene empogeditemcel (trem-cel; formerly known as VOR33) as a donor allograft for patients aged 18–70 years with CD33+ AML who have an elevated risk of relapse and are candidates for myeloablative conditioning allo-HSCT followed by gemtuzumab ozogamicin posttransplant maintenance therapy. Trem-cel is manufactured from patient-matched donor CD34+ cells and modified by clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (CRISPR/Cas9) gene editing to lack CD33. Following engraftment with trem-cel and recovery, patients received gemtuzumab ozogamicin maintenance therapy in a 3 + 3 dose escalation schedule from 0.5 to 2.0 mg/m2 every 28 days for 4–8 cycles. The primary endpoint was primary neutrophil engraftment by Day 28.
Key findings
- At the data cut-off of December 4, 2023, eight patients had been engrafted with trem-cel
- At Day 28, all patients had full myeloid chimerism
- Median time to neutrophil engraftment was 10 days
- Median time to platelet recovery was 15 days (excluding one patient with immune thrombocytopenia)
- Four patients received gemtuzumab ozogamicin maintenance therapy. In the three patients with sufficient follow-up:
- there was no significant decrease in neutrophil or platelet counts;
- no dose-limiting toxicities were reported;
- no increase in liver function tests above the upper limit of normal was observed, with no incidences of sinusoidal obstruction syndrome/veno-occlusive disease;
- the dose escalation committee recommended increasing the dose to 1 mg/m2; and
- after the first dose, mean Cmax was 236 ng/ml (standard deviation [SD], 151), and mean AUCinf (area from time of dosing extrapolated to infinity) was 10,890 Hr*ng/ml (SD, 13,958).
Presenter’s conclusion
All patients who received trem-cel demonstrated primary neutrophil engraftment. Gemtuzumab ozogamicin at 0.5 mg/m2 as maintenance therapy was well tolerated, and results indicate that gemtuzumab ozogamicin-related myelosuppression was prevented.
Conclusion
Several novel maintenance strategies for both the remission and posttransplant setting were presented at the 65th ASH Annual Meeting and Exposition, with promising initial results. These novel strategies have the potential to improve the poor outcomes associated with relapse in patients with AML.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




