All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
APR-246 plus azacitidine for patients with TP53 mutated MDS and AML
Patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) harboring TP53 mutations have poor prognosis. Response to treatment is generally short, and the risk of relapse following allogeneic stem cell transplantation (allo-SCT) is heightened in this subset of patients.
APR-246 reactivates mutant p53 via a number of mechanisms and has exhibited synergy with azacitidine (AZA) both in-vitro and in-vivo. Preclinical data have provided the rationale for clinical evaluation of the combination, and a phase II study conducted by the Groupe Francophone des Myélodysplasies (GFM) is currently underway. The GFM-APR trial (NCT03931291) is investigating the safety and efficacy of APR-246 plus AZA as maintenance therapy for patients with TP53 mutant AML or MDS following allo-SCT. Results were presented at this year’s virtual European Hematology Association (EHA) Annual Congress by Thomas Cluzeau.
Study design
- A total of 53 adult patients with TP35 mutated MDS (intermediate, high, and very high revised International Staging System [ISS-R] scores) or AML (including > 30% bone marrow blasts) were enrolled between September 2018 and July 2019 across seven GFM centers
- All patients were intended to receive six sessions of the following treatment regimen in 28-day cycles:
- APR-246 4,500 mg intravenous (IV)/day (6-hour infusions; days 1–4)
- AZA 75 mg/m²/day (days 4–10)
- Allo-SCT was advised following 3–6 treatment cycles, followed by maintenance treatment with
- APR-246 3,700 mg IV/day (6-hour infusions; days 1–4)
- AZA 36 mg/m²/day (days 1–5)
Endpoints
- Primary endpoint: Response of intention to treat and all evaluable patients* determined using the International Working Group (IWG) 2006 and 2003 criteria for MDS and AML, respectively
- Secondary endpoints: Safety, overall survival (OS), duration of response (DoR), AML disease progression, and correlation of TP53 variant allele frequency with p53 expression
*Patients who received ≥ 3 cycles and underwent bone marrow (BM) evaluation
Results
- A total of 52 patients were enrolled by July 2019 (Table 1)
- Data cutoff: April 1, 2020
Table 1. Baseline patient characteristics
|
AML, acute myeloid leukemia; IPSS-R, revised International Prognostic Scoring System; MDS, myelodysplastic syndromes, WHO, World Health Organization |
||
|
Total patients (N = 52) |
MDS (% of patients) (n = 34) |
AML (% of patients) (n = 18) |
|
Median age, years (range) |
74 (46–87) |
72 (44–83) |
|
Male/Female |
15/19 |
12/6 |
|
WHO 2016 classification, % |
|
|
|
MDS IPSS-R |
|
|
|
Intermediate |
12 |
– |
|
High |
15 |
– |
|
Very high |
74 |
– |
|
AML |
|
|
|
20–30% blasts |
– |
61 |
|
> 30% blasts |
– |
39 |
|
Cytogenetic risk, % |
|
|
|
Complex karyotype |
85 |
89 |
|
Monosomal karyotype |
79 |
50 |
|
del(5q) |
53 |
67 |
Efficacy
- Overall response rates (ORRs) in patients who received APR-246 + AZA are shown in Figure 1
- ORR in all evaluable patients (BM evaluated after at least three cycles) according to the WHO classification was 75% in MDS, 78% in AML < 30 blasts, 100% AML > 30 blasts
- OS rates were superior in patients who received ≥ 3 cycles of APR-246 + AZA (Table 2)
- Median follow-up was 9.7 months
Figure 1. ORRs at A varying analysis timepoints and B according to WHO disease classification
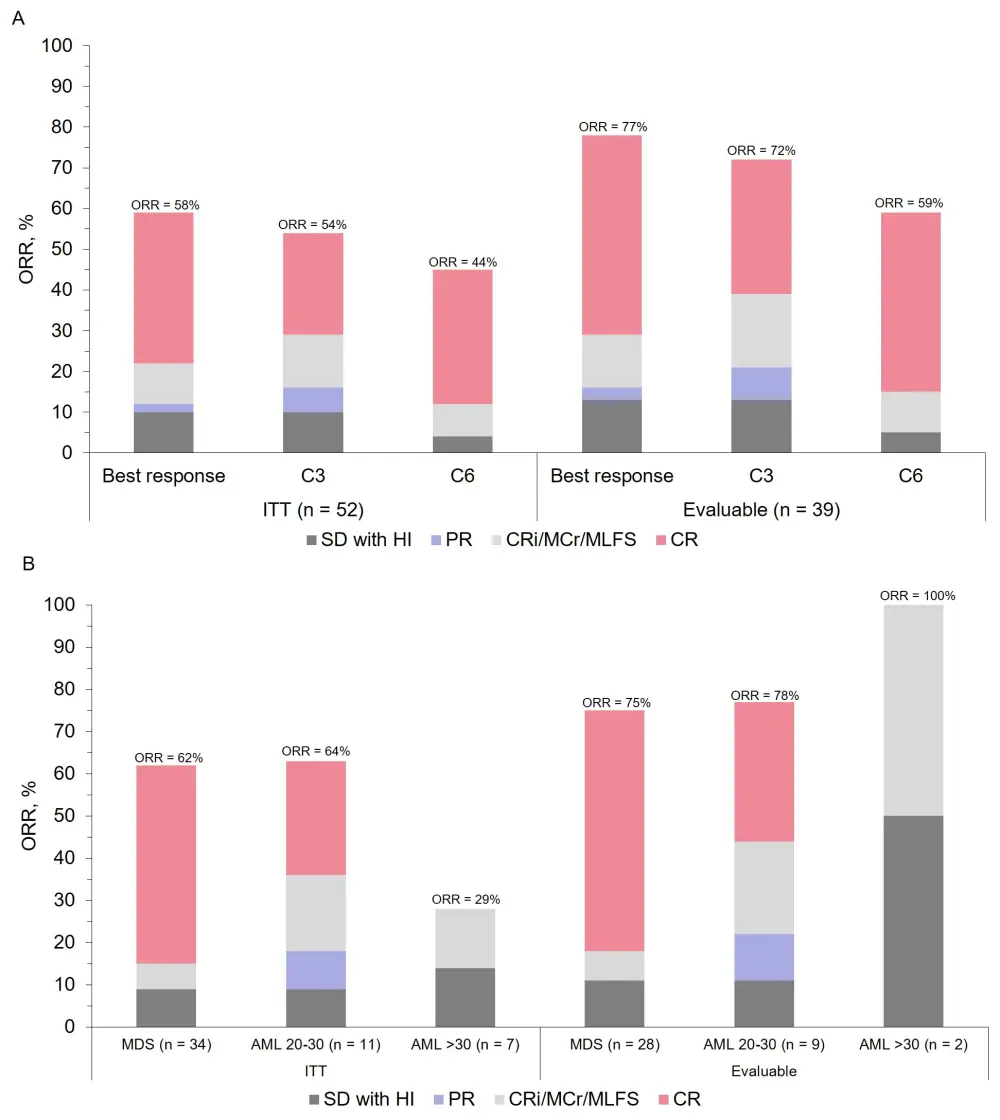
CR, complete response; CRi, CR with incomplete count recovery; HI, hematologic improvement; PR, partial response; SD, stable disease; WHO, World Health Organization
Table 2. Patient OS rates
|
AML, acute myeloid leukemia; ITT, intention to treat; MDS, myelodysplastic syndrome; OS, overall survival |
|||
|
OS |
Median OS, months |
95% CI |
P |
|
ITT cohort |
12.1 |
8.9–15.3 |
|
|
Disease |
|
|
0.34 |
|
MDS |
12.1 |
8.9–15.3 |
|
|
AML 20–30% blasts |
13.9 |
5.4–22.5 |
|
|
AML > 30% blasts |
3.0 |
0.0–6.5 |
|
|
Treatment duration |
|
|
<0.0001 |
|
≥ 3 cycles |
13.7 |
11.7–15.7 |
|
|
< 3 cycles |
2.8 |
1.2–4.4 |
|
TP53 mutations in evaluable patients
- 50 TP53 mutations were sequenced in 39 patients
- The vast majority were in the DNA-binding domain (92%)
- 95% patients had at least one mutation in this domain
- 51% of patients achieved TP53 negativity by next generation sequencing
- In responders TP53 negativity reached significance after Cycle 3 (p < 0.0001)
Safety
- Grade 3–4 adverse events (AEs) occurring in patients who were treated with APR-246 + AZA are shown in Table 3
- Neurological toxicities were associated with lower glomerular filtration rate (p < 0.01) and older age (p = 0.01), and were manageable, reversible, and non-recurring following dose reduction
- In total, 13 patients discontinued treatment prior to Cycle 3 analysis due to
- severe infection (n = 6)
- AML progression (n = 4)
- multiorgan dysfunction (n = 2)
- withdrawal of consent (n = 1)
- One early discontinuation was secondary to an APR-246 adverse event
- Patient mortality at 30 and 60 days were 0 and 8%, respectively
Table 3. Grade 3–4 AEs
|
Grade 3–4 AE |
% of global cohort (N = 52) |
|
Febrile neutropenia |
37 |
|
Neurological |
6 |
|
Ataxia |
4 |
|
Acute confusion |
2 |
|
AE, adverse event |
|
Conclusions
Results from this trial indicate that the combination of APR-246 and AZA possesses encouraging safety and efficacy profiles in patients with TP53-mutated MDS and AML. The study highlighted subgroups of patients (elderly and those with renal failure) who may be particularly susceptible to neurologic side effects. Nonetheless, all AEs were manageable and reversible, and therefore close monitoring of these patients is recommended.
These data could contribute towards a more efficacious treatment route for patients with TP53 mutant disease whose options are currently limited.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

