All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Venetoclax in combination with hypomethylating agents for the treatment of older patients with NPM1-mutated acute myeloid leukemia
Approximately 30% of patients with acute myeloid leukemia (AML) have nucleophosmin-1 mutations (NPM1+), which are associated with a favorable prognosis.1 However, the beneficial impact of this mutation decreases with increasing age in patients treated with standard intensive chemotherapy (IC) or hypomethylating agents (HMAs).2 Newer studies have also shown venetoclax (VEN)-based treatment regimens to be efficacious in the treatment of NPM1+ AML.3,4
Curtis A. Lachowiez and colleagues have recently published the results of a retrospective study in the journal Blood Advances, which compares HMA plus VEN, HMA monotherapy, or IC treatment regimens for older patients (> 65 years) with NPM1+ AML.5
Study Design
- Eligible patients were identified as those with a diagnosis of NPM1+ AML who had received frontline AML therapy at the MD Anderson Cancer Center between 2007 and 2019
- Patients were stratified into cohorts based on the per-protocol induction therapy received: HMA plus VEN, HMA, or IC (defined as receipt of cytarabine plus anthracycline)
- Patients treated with IC in combination with targeted therapeutics (fms-like tyrosine kinase 3 [FLT3], isocitrate dehydrogenase 1 and 2 [IDH1, IDH2] inhibitors) were included, and additional chemotherapeutic agents such as fludarabine or cladribine were allowed
- HMA cohort included those treated with HMA monotherapy (azacitidine or decitabine) or with the combination of an FLT3 inhibitor (FLT3i)
- The aim of this study was to retrospectively evaluate the outcomes of patients with NPM1+ AML including:
- Response to therapy, assessed using the European LeukemiaNet criteria
- Overall survival (OS), calculated as time from the start of induction therapy to the date of death or last follow-up
- Measurable residual disease (MRD) status in composite complete response (CRc; complete response [CR] + CR with incomplete count recovery [CRi]) by 8-color multiparameter flow cytometry
Patient characteristics
- Of a total of 446 patients with NPM1+ AML identified, 303 were included in the analysis as seen in Figure 1 (n = 28 HMA plus VEN; n = 47 HMA; n = 228 IC)
Figure 1. Percentage of patients included in each cohort
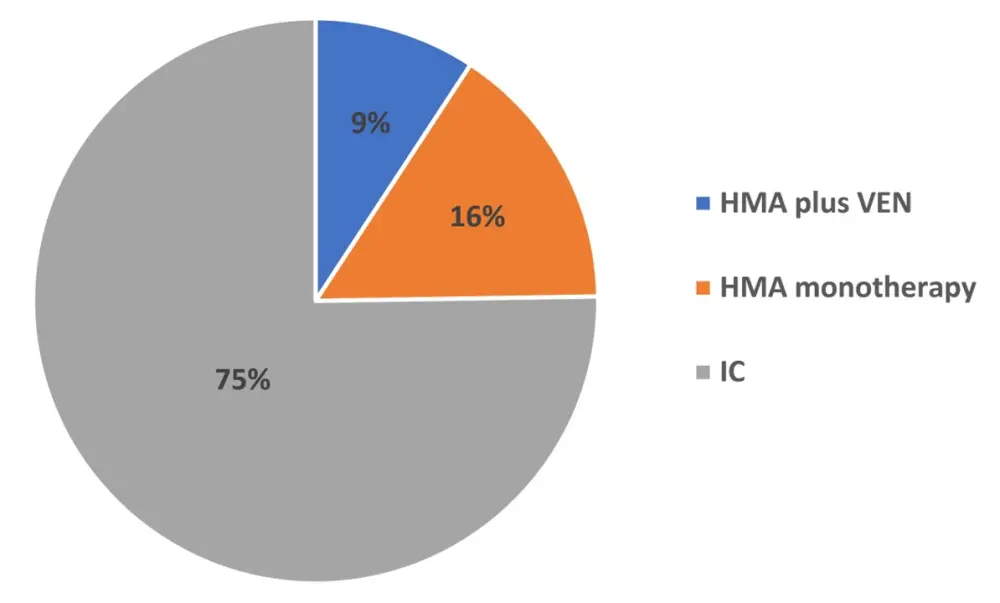
HMA, hypomethylating agents; IC, intensive chemotherapy; VEN, venetoclax
- 143 patients were excluded as they received induction regimens that could not be classified into one of the three treatment regimens
- The IC cohort had a higher representation of younger patients compared with the HMA or HMA plus VEN cohort (median ages: 55, 72, and 71 years respectively; Table 1)
Table 1. Baseline patient demographics
|
AML, acute myeloid leukemia; HMA, hypomethylating agents; IC, intensive chemotherapy; VEN, venetoclax *HMA plus VEN vs IC Data are n |
||||
|
|
HMA plus VEN (n = 28) |
HMA (n = 47) |
IC (n = 228) |
p |
|---|---|---|---|---|
|
Median age, years |
71 |
72 |
55 |
0.0001 |
|
< 55 |
- |
- |
114 |
|
|
55–65 |
2 |
8 |
89 |
|
|
> 65 |
26 (median, 72) |
39 (median, 75) |
25 (median, 68) |
0.0040* |
|
Performance status |
|
|
|
|
|
0–1 |
15 |
23 |
180 |
0.0002 |
|
2–3 |
9 |
13 |
27 |
|
|
AML subtype |
|
|
|
|
|
De novo |
25 |
35 |
213 |
0.0010 |
|
Secondary |
1 |
6 |
4 |
- |
|
Treated secondary |
2 |
6 |
11 |
- |
Results
- CRc rates at 1-year median follow-up were 96%, 36%, and 89% of HMA plus VEN, HMA, and IC patients, respectively
- CR rates stratified by age (Table 2), showed that patients aged > 65 years with NPM1+ AML, treated with HMA plus VEN achieved a high CR rate compared with patients receiving IC regimens
Table 2. Treatment outcomes
|
CR, complete response; HMA, hypomethylating agents; IC, intensive chemotherapy; MRD, measurable residual disease; VEN, venetoclax *HMA plus VEN vs HMA †HMA plus VEN vs IC |
||||
|
|
HMA plus VEN (n = 28) |
HMA (n = 47) |
IC (n = 228) |
p |
|---|---|---|---|---|
|
% CR by age, y |
|
|
|
|
|
< 55 |
— |
— |
89 |
— |
|
55–65 |
100 |
13 |
88 |
0.067* 1.000† |
|
> 65 |
88 |
28 |
56 |
<0.001* 0.013† |
|
Total |
89 |
26 |
85 |
<0.001* 0.778† |
|
MRD negativity |
75 |
27 |
79 |
0.011* 0.593† |
- When patients of all ages were included in the analysis, OS was not significantly different between HMA plus VEN compared with IC (median OS, not reached [NR] vs 3.7 years respectively; p = 0.292)
- In patients aged > 65 years, HMA plus VEN demonstrated a significant improvement in OS compared with IC (median OS, NR vs 0.9 years; p < 0.001). Furthermore, HMA plus VEN outperformed HMA monotherapy (median OS, NR vs 0.4 years; p < 0.001).
- After a median follow-up of 1 year, 80% of patients age > 65 years treated with HMA plus VEN survived, compared with 36% of patients treated with IC and 12% of patients treated with HMA
- MRD analysis by flow cytometry showed that MRD negativity was achieved in a substantial portion of patients treated with HMA plus VEN, compared with HMA monotherapy (75% vs 27%, respectively; Table 2), indicating this regimen can induce deep remissions
Conclusions
These results provide further evidence of the improved outcomes of using HMA combinations over HMA monotherapy as a treatment for older patients with NPM1+ AML. However, the authors acknowledge that limitations of this study include its retrospective nature, as well as small sample sizes for the HMA plus VEN, and HMA cohorts.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

