All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
The latest on venetoclax combinations in AML from ASH 2021
Your opinion matters
How would you treat relapsed AML patients following ven+aza based therapy?
The BCL-2 inhibitor, venetoclax, has transformed the treatment landscape for acute myeloid leukemia (AML), particularly in cases where patients are considered ineligible for intensive chemotherapy. This agent has demonstrated promising responses when used alongside low-dose cytarabine and hypomethylating agents in this patient population, resulting in the widespread evaluation of venetoclax in alternative settings and as the backbone of different combination therapies.1
Over the coming months, the AML Hub will be focusing on venetoclax combinations in the AML setting as an educational theme. In this introductory article, we provide a summary of the emerging data across abstracts relevant to this topic from the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition.
Venetoclax + decitabine
DEC10-Ven: Genomic subgroup analysis (#694)2
Encouraging responses have been observed with the DEC10-Ven (10-day decitabine + venetoclax) regimen in older patients with newly diagnosed (ND) or relapsed/refractory (R/R) AML. However, resistance to venetoclax has been reported in patients with genomic mutations in TP53, FLT3, and RAS; although long-term outcomes in these patients require further evaluation. At ASH 2021, Abhishek Maiti presented the updated outcomes of patients receiving DEC10-Ven in a single-center, phase II clinical trial, with a focus on genomic subgroups. Of the 199 patients enrolled, 83 had ND AML, 20 untreated secondary AML, 25 treated secondary AML, and 71 R/R AML.
Responses to DEC10-Ven according to genomic subgroup are presented in Figure 1 and other outcomes are in Table 1. Responses were particularly promising in patients who had not received prior treatment, which was maintained across all subgroups (Figure 1A), and in patients with NPM1- or IDH1/1- mutated AML, regardless of prior therapy.
Figure 1. Patient responses to DEC10-Ven by genomic subgroup in A treatment-naïve patients and B previously treated patients*
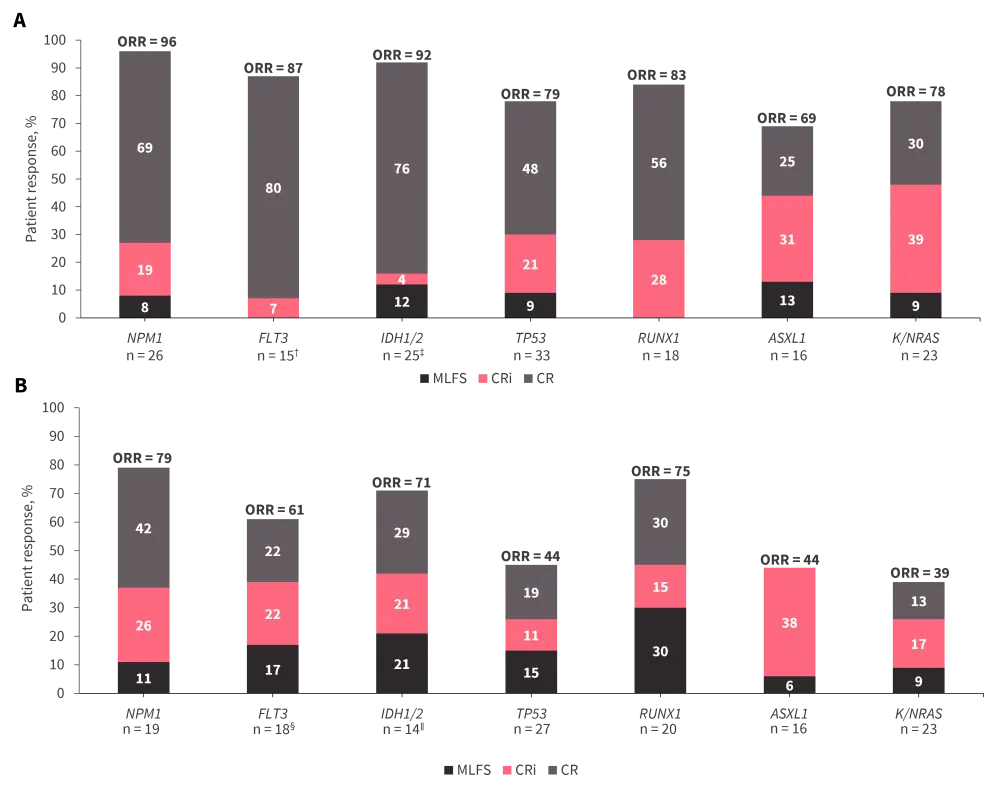
CR, complete remission; CRi, CR with incomplete hematologic recovery; MLFS, morphologic leukemia-free state; ORR, overall response rate.
*Data from Maiti, et al.2
†70% of FLT3mut patients received tyrosine kinase inhibitor therapy.
‡Two patients received enasidenib at treatment initiation and after Cycle 15.
§95% of FLT3mut patients received tyrosine kinase inhibitor therapy.
∥One patient received enasidenib after Cycle 6.
Table 1. Patient outcomes to DEC10-VEN by genomic subgroup and prior therapy*
|
AML, acute myeloid leukemia; IE, inevaluable; MRD, measurable residual disease; NR, not reached OS, overall survival; RFS, relapse-free survival. |
|||||||
|
Treatment-naïve AML |
NPM1 |
FLT3 |
IDH1/2 |
TP53 |
RUNX1 |
ASXL1 |
K/NRAS |
|---|---|---|---|---|---|---|---|
|
MRD negative† |
91 |
82 |
90 |
50 |
62 |
55 |
64 |
|
No response/ failure / IE, % |
4 |
14 |
8 |
21 |
17 |
31 |
22 |
|
Median OS, months |
NR |
24.5 |
29.6 |
5.4 |
16.2 |
15.2 |
12.1 |
|
Median RFS, months |
NR |
20.0 |
NR |
3.1 |
9.7 |
8.5 |
6.5 |
|
Previously treated AML |
NPM1 |
FLT3 |
IDH1/2 |
TP53 |
RUNX1 |
ASXL1 |
K/NRAS |
|
MRD negative† |
87 |
70 |
70 |
37 |
60 |
71 |
56 |
|
No response / failure / IE, % |
16 |
37 |
29 |
56 |
25 |
56 |
61 |
|
Median OS, months |
12.4 |
6.6 |
16.9 |
4.5 |
13.7 |
9.0 |
6.0 |
|
Median RFS, months |
15.6 |
6.6 |
15.3 |
9.4 |
12.9 |
10.7 |
18.9 |
Venetoclax + decitabine for adverse-risk AML in young adults (#35)3
Young, fit adults with AML generally demonstrate good complete remission (CR) rates following induction therapy with the 7+3 cytarabine and anthracycline regimen. However, suboptimal responses have been observed in fit patients who are considered ‘adverse-risk’ compared with those with favorable- and intermediate-risk disease. Although established in elderly patients, the clinical impact of venetoclax + decitabine induction therapy for the treatment of young adults with AML is ill-defined.
Suning Chen presented data from an interim analysis of a phase II, multicenter study (NCT04752527) evaluating venetoclax + decitabine in young adults (median age, 40 years) with ND adverse-risk AML (European LeukemiaNet recommendations). Compared with a historical control, venetoclax + decitabine demonstrated encouraging patient responses, including composite CR rates, after one (Figure 2A) and two (Figure 2B) cycles of therapy.
Low incidences of pneumonia, sepsis, and intestinal infection were observed in the venetoclax + decitabine cohort compared with the historical cohort (8.0% vs 46.7%). The 60-day mortality rate was 0% and median OS was not reached.
Figure 2. Patient responses to venetoclax + decitabine versus historical control following A one cycle of therapy and B two cycles of therapy*
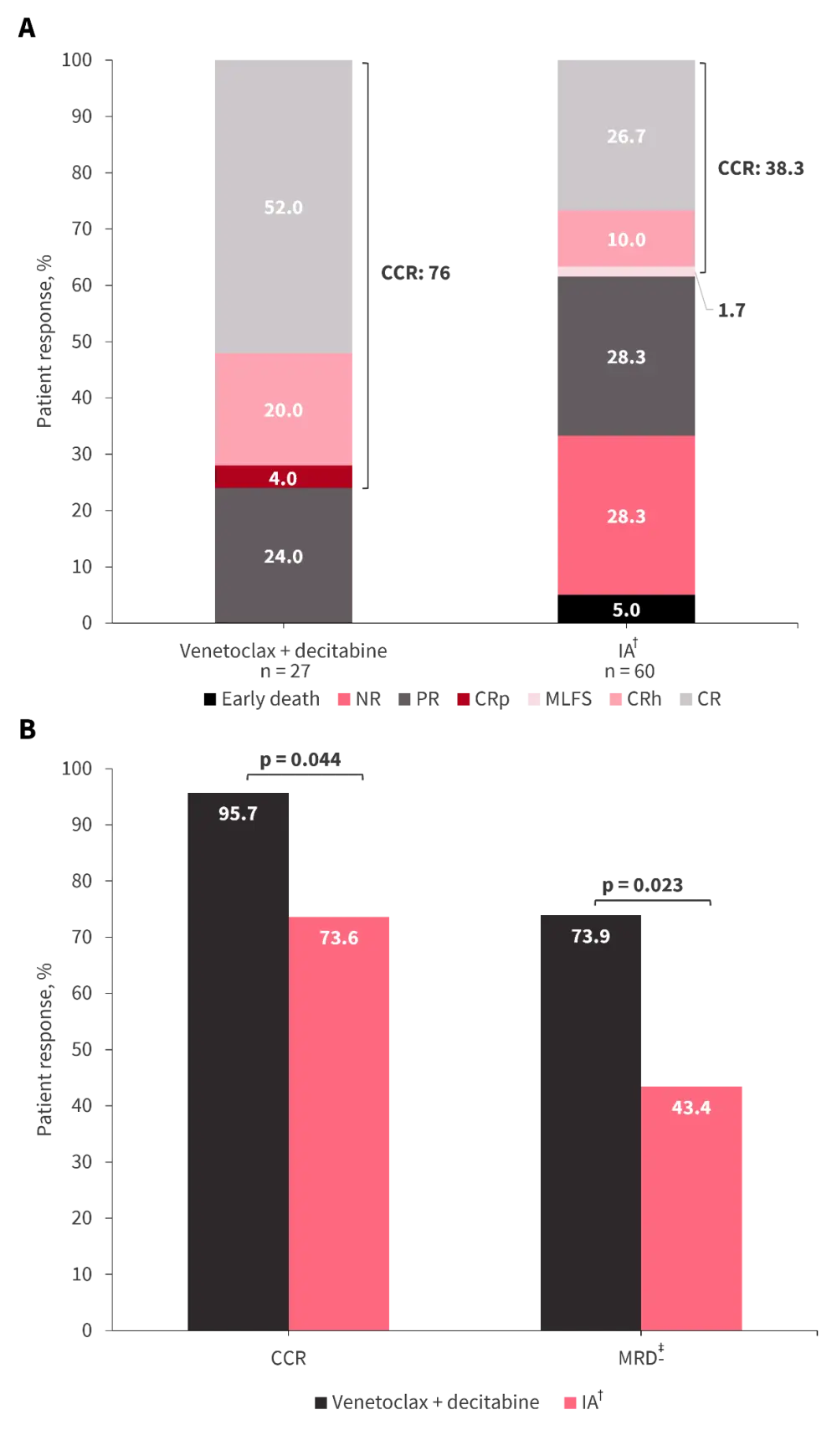
CCR, composite complete remission; CR, complete remission; CRh, CR with partial hematological recovery; CRp, CR with incomplete platelet recovery; IA, cytarabine + idarubicin; MLFS, morphologic leukemia-free state; MRD, measurable residual disease; NR, non-responder; PR, partial remission.
*Adapted from Chen, et al.3
†Cytarabine + idarubicin (12 mg/m2).
‡MRD negativity defined as MRD <10−3.
Venetoclax + magrolimab (#371)4
The anti-CD47 monoclonal antibody, magrolimab, promotes AML blast phagocytosis via inhibition of CD47 on macrophages. In patient-derived xenograft preclinical AML models, this translates to prolonged survival in both azacitidine/venetoclax-sensitive and -refractory subjects. It has been hypothesized that the combination of magrolimab with azacitidine and venetoclax may improve long-term outcomes in patients who relapse after front-line therapy and ND patients with TP53-mutated AML, populations with otherwise poor prognosis.
At ASH 2021, Naval Daver outlined initial data from a phase Ib/II trial (NCT04435691) evaluating azacitidine plus venetoclax with magrolimab in patients aged ≥18 years. Initial enrollment to the phase Ib portion was restricted to patients with R/R AML, before being extended to include treatment-naïve patients and those with venetoclax-naïve or -exposed R/R AML.
Particularly encouraging CR rates were noted in the ND cohort, of whom 82% were classed as adverse risk, irrespective of TP53 mutation status (Figure 3A). Suboptimal responses were observed in the venetoclax-exposed R/R cohort (Figure 3B), with a high rate of mortality before 6 months. The 8-week mortality rate was 0% in the frontline-treatment cohort, and 13% and 20% in venetoclax-naïve and -exposed R/R cohorts, respectively.
Figure 3. Responses to magrolimab with azacitidine and venetoclax in A treatment-naïve patients and B patients with R/R AML*
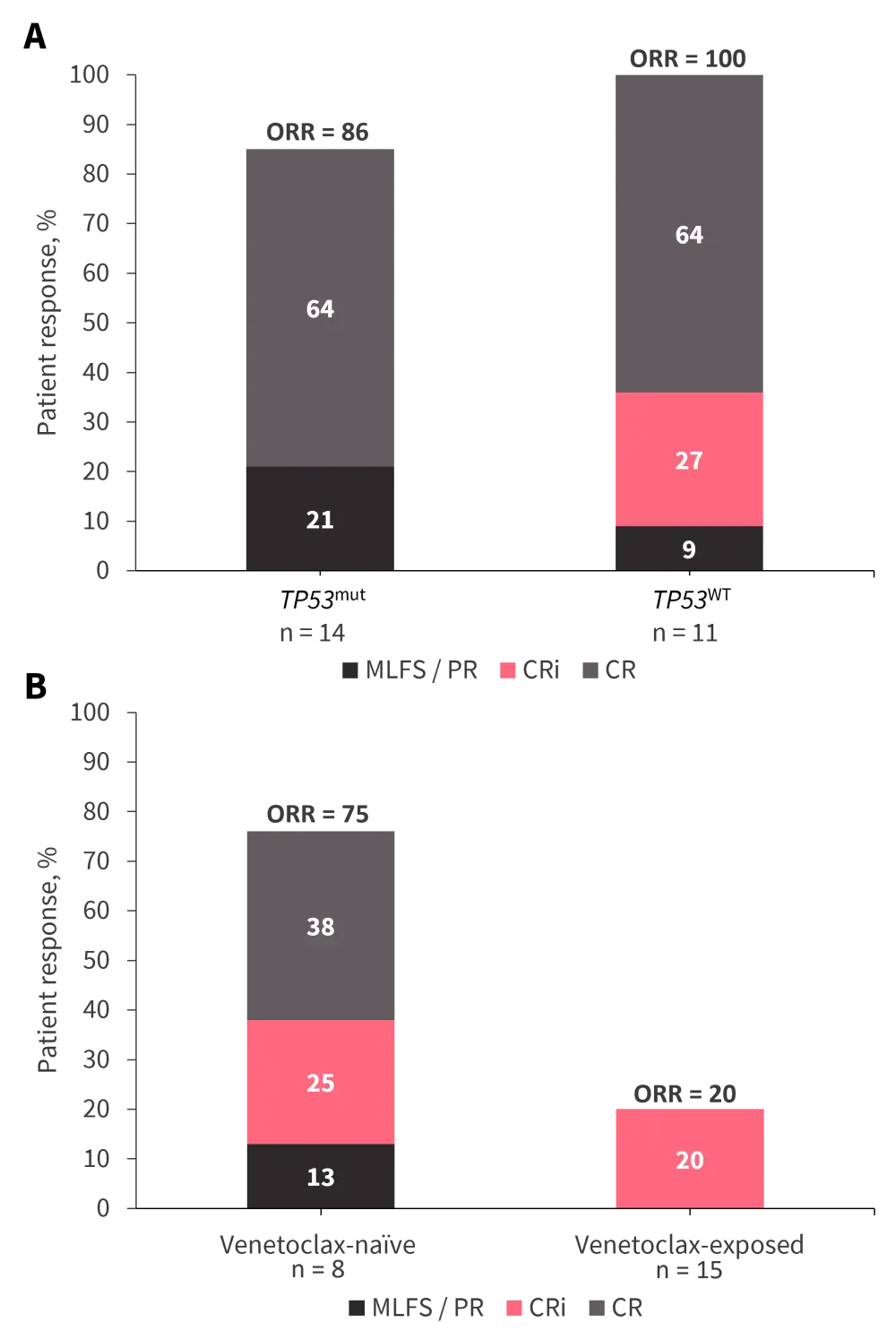
CR, complete remission; CRi, CR with incomplete hematologic recovery; MLFS, morphologic leukemia-free state; ORR, overall response rate; PR, partial response.
*Data from Daver, et al.4
Naval Daver highlighted the impressive absolute neutrophil count and platelet recovery in the treatment-naïve cohort, particularly considering the notable myelosuppression associated with other triplet combination therapies. There were no treatment immune-related adverse events recorded in patients receiving magrolimab with azacitidine and venetoclax, nor were there any discontinuations due to treatment-related adverse events.
IMGN632 with venetoclax + azacitidine for CD123-positive AML (#372)5
Overexpression of the IL-3 receptor alpha subunit CD123 is typical of both mature AML blasts and putative leukemia stem cells, but not hematopoietic stem cells. Inhibition of CD123 has demonstrated synergy with venetoclax and azacitidine in pre-clinical murine AML models and may help to address drug resistance.
The CD123-directed antibody-drug conjugate, IMGN632, has been evaluated with both azacitidine and venetoclax independently in the AML setting. At ASH 2021, Naval Daver presented the findings of a phase Ib/II study investigating the efficacy and safety of IMGN632 in combination with azacitidine and venetoclax for the treatment of patients with CD123+ AML. Of the 51 patients enrolled, 49% had adverse risk disease.
Several dosing regimens were evaluated; patients who received higher intensity dosing (IMGN632 on Day 7 at the higher dose of 45 mcg/kg or venetoclax for 14/21 days or both) demonstrated greater response rates (Figure 4A). Subset analyses of the higher intensity cohort identified groups of patients who may benefit from the novel triplet combination therapy (Figure 4B).
Figure 4. Responses to IMGN632 with azacitidine and venetoclax in A the efficacy evaluable population versus higher intensity dosing cohort and B patient subsets of the higher intensity dosing cohort*
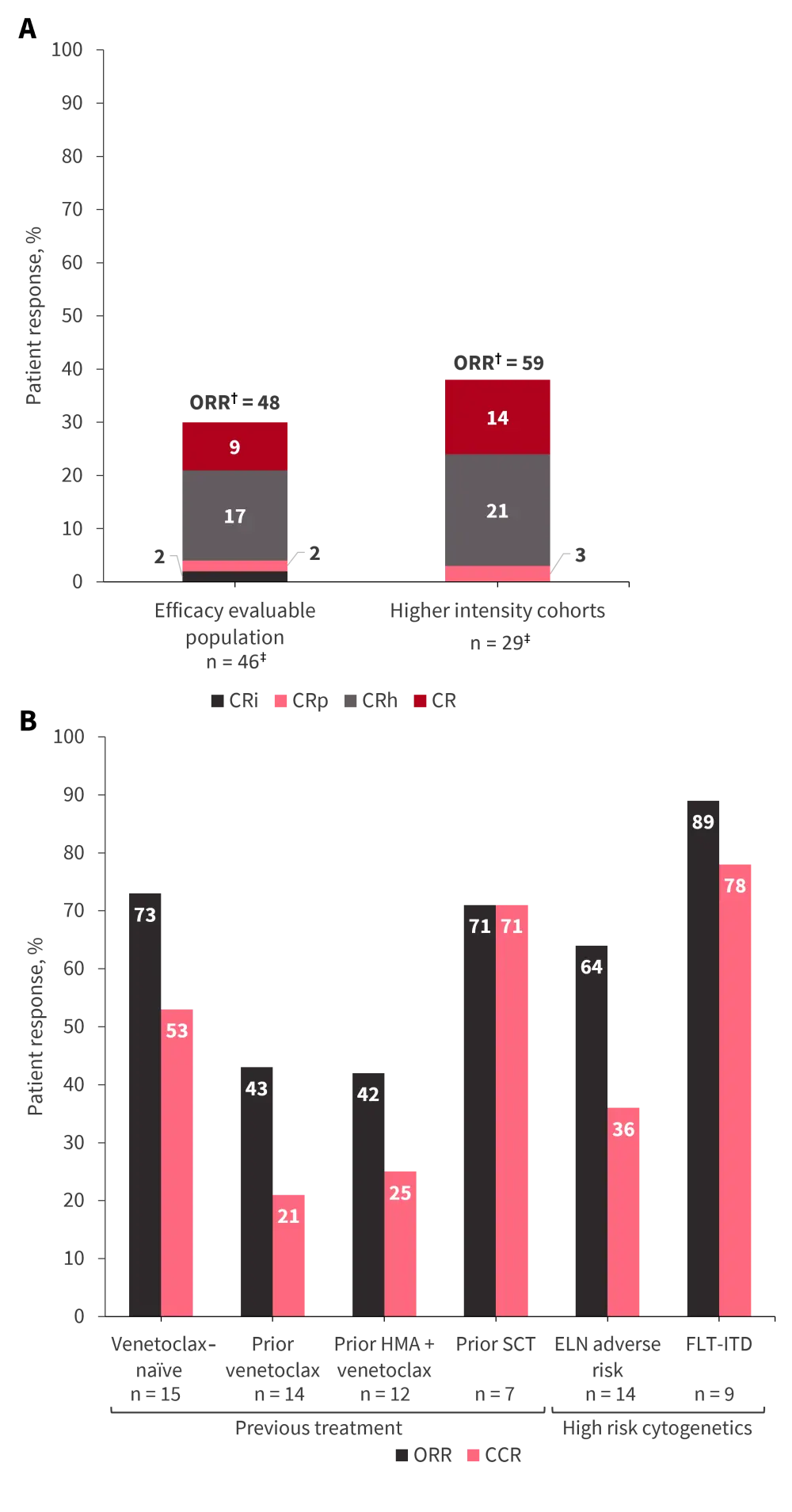
CCR, composite complete remission; CR, complete remission; CRh, CR with partial hematologic recovery; CRi, CR with incomplete hematologic recovery; CRp, CR with incomplete platelet recovery; ELN, European LeukemiaNet; HMA, hypomethylating agent; ITD, internal tandem duplication; ORR, overall response rate; SCT, stem cell transplant.
*Data from Daver, et al.5
†ORR = CR +CRh + CRp + CRi + morphologic leukemia free state (data not provided).
‡At the time of analysis, five patients, including three in the high intensity cohort, were in their first cycle of treatment and were excluded.
Adverse events were consistent with previously reported azacitidine- and venetoclax-based regimens, with no new safety concerns. Mortality rates at 30 days and 60 days were 0% and 10%, respectively, and discontinuation due to adverse events was 8%.
The dose escalation portion of the study is ongoing and phase II expansion is planned for evaluation of IMGN632 with azacitidine and venetoclax in the R/R and frontline settings (NCT04086264).
Conclusion
A number of exciting abstracts concerning venetoclax combination therapies were presented at the 63rd ASH Meeting and Exposition. Taken altogether, these data suggest that venetoclax-based regimens could represent a promising treatment option for patient populations with historically poor prognosis.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

