All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Survival outcomes in older patients receiving CPX-351 versus HMA + venetoclax as frontline AML therapy
New frontline therapy options have recently improved the acute myeloid leukemia (AML) treatment landscape for older patients, including the liposomal formulation of daunorubicin and cytarabine (CPX-351) and hypomethylating agent (HMA) + venetoclax (HMA + Ven) combination. CPX-351 is approved by the U.S. Food and Drug Administration (FDA) for the treatment of newly diagnosed (ND) AML, secondary and therapy-related AML in older fit patients, and, more recently, pediatric patients (<1 year old). Meanwhile, HMA + Ven is approved by the FDA for the treatment of ND AML in older patients who are ineligible for intensive chemotherapy, and those with biologically adverse disease regardless of fitness. Despite the different indications, the two regimens have been used in increasingly similar cohorts due to challenges in defining patient fitness.1
At the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, Justin Grenet presented results of a large, multicentre, retrospective study comparing characteristics and outcomes of patients receiving CPX-351 or HMA + Ven.1 We summarise key findings below.
Study design
Data were retrospectively collected from four large academic centres: Memorial Sloan Kettering Cancer Center, Northwestern University, Moffitt Cancer Center, and Weill Cornell Medicine, US. The overall study design, including patient cohort numbers and primary outcomes, are summarized in Figure 1.
Figure 1. Study design*
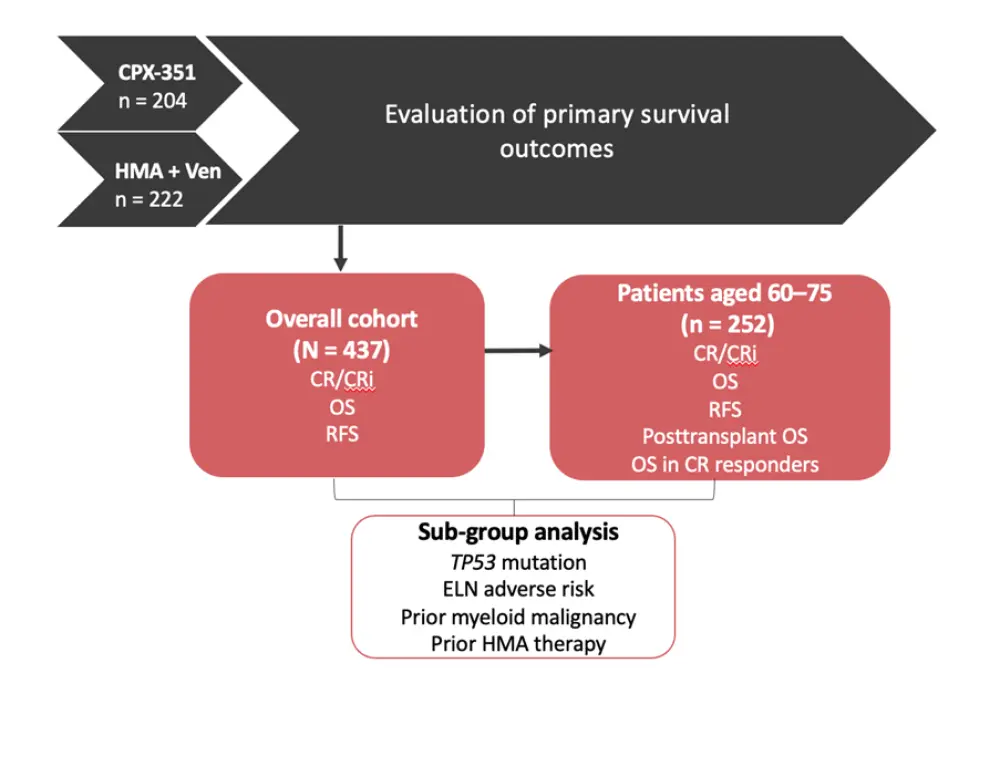
CR, complete remission; CRi, complete remission with incomplete hematologic recovery; ELN, European leukemiaNet; HMA, hypomethylating agent; OS, overall survival; RFS, relapse-free survival; Ven, venetoclax.
*Adapted from Grenet et al.1
Results
Patient characteristics for the CPX-351 and HMA + Ven cohorts are shown in Table 1. There were several significant differences, including a greater proportion of older patients (p < 0.001), those classified as European leukemiaNet (ELN) adverse risk, and those carrying ASXL1 mutations (p = 0.01) in the HMA + Ven cohort compared to CPX-351. Prior myeloid malignancy (p = 0.005) and prior HMA treatment (p = 0.001) were more common in patients receiving CPX-351 vs HMA + Ven.
Table 1. Patient characteristics*
|
ELN, European leukemiaNet; HMA, hypomethylating agent; Ven, venetoclax. |
||
|
Characteristic |
CPX-351 |
HMA + Ven |
|---|---|---|
|
Age, median (range) |
66.8 (60.8–71.6) |
75.2 (69.7–78.8) |
|
Male, % |
57.4 |
61.1 |
|
ELN risk, % |
||
|
Favorable/intermediate |
38.9 |
28.3 |
|
Adverse |
61.1 |
71.7 |
|
Mutational status, % |
||
|
TP53 |
19.1 |
26.7 |
|
FLT3 |
6.1 |
8.9 |
|
NPM1 |
6.7 |
10.7 |
|
RUNX1 |
22.7 |
24.9 |
|
ASXL1 |
16.5 |
27.1 |
|
IDH1/IDH2 |
19.7 |
18.4 |
|
Prior myeloid malignancy, % |
54.0 |
40.7 |
|
Prior HMA therapy, % |
20.4 |
9.7 |
Survival outcomes
Overall, there was no difference in combined rates of complete remission (CR)/CR with incomplete hematologic recovery (CRi) between patients treated with CPX-351 (57.8%) and HMA + Ven (56.6%; p = 0.803). Combined CR/CRi rates were also similar between mutational subgroups. However, median overall survival (OS) was longer in the CPX-351 cohort compared with HMA + Ven (17.3 months vs 11.1 months; p = 0.007), although no difference in relapse-free survival was reported.
In those patients classified with intermediate/favorable risk AML, there was no difference in OS between treatment groups; however, patients with adverse risk treated with CPX-351 survived longer than those treated with HMA + Ven (p = 0.0392). Grenet highlighted that such differences may be driven by the higher rates of hematopoietic stem cell transplant (HSCT) in the younger CPX-351 group.1
Multivariate analysis, adjusted for age, ELN risk, prior myeloid malignancy, and prior HMA therapy, revealed improved survival in patients receiving CPX-351 in the overall population, and also after stratification by TP53 mutation status, prior myeloid malignancy, and ELN adverse risk (Table 2).
Table 2. Multivariate analysis*
|
CI, confidence interval; ELN, European leukemiaNet; HMA, hypomethylating agent; HR, hazard ratio. |
||
|
Overall survival |
HR (95% CI) |
p value |
|---|---|---|
|
Overall cohort |
0.742 (0.553–0.955) |
0.046 |
|
TP53 mutation |
0.406 (0.224–0.735) |
0.003 |
|
Prior myeloid malignancy |
0.640 (0.440–0.931) |
0.02 |
|
Prior HMA use |
0.489 (0.276–0.866) |
0.014 |
|
ELN adverse risk |
0.668 (0.482–0.925) |
0.015 |
Survival outcomes in patients aged 60–75
Within patients aged 60–75 years, CR/CRi and OS rates were similar between the two treatment cohorts, despite HSCT being more common in the CPX-351 vs HMA + Ven (47.7% vs 19%; p < 0.001). Additionally, survival did not favor either treatment when analyzing patients by ELN risk.
Multivariate analysis adjusted for age, ELN risk, prior myeloid malignancy, and prior HMA therapy, did not reveal any differences in survival between treatment cohorts or transplantation status. However, survival was improved in patients carrying TP53 mutations and treated with CPX-351 (HR, 0.395; 95% CI, 0.191–0.820; p = 0.013).
In the subgroup of patients in this age group who achieved CR/CRi, there was no survival difference between treatment cohort. Likewise, post-transplant survival rates were similar.
Grenet also presented preliminary data from an ongoing comorbidity analysis (CPX-351, n = 31; HMA + Ven, n = 58) which showed higher rates of nonzero Ferrara scores in the HMA + Ven group (24.1%) than the CPX-351 group (6.5%; p = 0.045), suggesting around 75% of patients receiving HMA + Ven were considered fit.1 Notably, survival in patients with a pre-induction Ferrara comorbidity score of 0 was similar regardless of treatment group.
Conclusion
This first real-world comparison of characteristics and outcomes in patients receiving frontline CPX-351 and HMA + Ven found response rates to be similar. While survival was improved in the CPX-351 group, it is likely that higher transplant rates in these patients contributed to this difference. In older patients (aged 60–75), survival appeared comparable between the two therapies, despite the higher rate of transplantation in the CPX-351 cohort.
Study limitations highlighted in this presentation included the inherent biases of retrospective chart reviews, lack of measurable residual disease data, and small patient numbers limiting post-transplant analysis.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

