All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Role of MRD in treatment decisions for AML: Latest insights from ASH 2023
The presence of measurable residual disease (MRD) in patients with acute myeloid leukemia (AML) following intensive chemotherapy is associated with poor outcomes.1 NPM1 MRD detected by real-time quantitative polymerase chain reaction (RT-qPCR) following two courses of induction is a robust prognostic marker.2 Similarly, MRD has prognostic utility in the pretransplant setting.3,4,5,6 Patients MRD status postinduction chemotherapy may be useful to guide treatment decisions.1,4
During the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, several presentations discussed the prognostic utility of MRD in patients with AML, and how this can influence treatment decisions. Below we summarize presentations from Russel.1, Othman.2,4, Loo.3, and Gui.5,6 discussing the role of MRD in treatment decisions.
MRD-directed treatment intensification is associated with survival benefit1
The AML Hub previously reported the study design of the UK AML18 (NCT02272478). Following the first course of induction chemotherapy, eligible patients included those who had:
- no complete remission (CR) or CR with incomplete count recovery (CRi);
- CR/CRi with MRD positive (MRD+) status; and
- CR/CRi with MRD status unknown.
For course 2 of the induction therapy, patients were randomized to either continue treatment with standard daunorubicin + cytarabine (DA) or receive up to two courses of intensified chemotherapy with either fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin (FLAG-Ida) or DA with cladribine (DAC). In total, 523 patients were randomized (193 to DA, 191 to FLAG-Ida, and 139 to DAC). The median age was 67 years, and patient characteristics were similar between treatment arms.
Key findings
- Count recovery time and supportive care requirements were higher in the intensification arms (Table 1).
Table 1. Count recovery time and supportive care requirements by treatment type in the AML18 trial*
|
|
DA |
FLAG-Ida |
DAC |
p-value |
|---|---|---|---|---|
|
Blood transfusions, median units |
5 |
8 |
7 |
< 0.001 |
|
Platelet transfusion, median units |
4 |
8 |
7 |
< 0.001 |
|
Days to ANC > 1.0 × 109/L, median |
25 |
30 |
29 |
< 0.001 |
|
Days to platelets > 100 × 109/L, median |
26 |
34 |
33 |
< 0.001 |
|
6 |
13 |
11.5 |
< 0.001 |
|
|
Nights in hospital |
24 |
32 |
29 |
< 0.001 |
|
ANC, absolute neutrophil count; DA, daunorubicin + cytarabine; DAC, DA with cladribine; FLAG-Ida, fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin; IV, intravenously. *Adapted from Russel.1 |
||||
- The early death rate at Day 30 and Day 60 were higher in the FLAG-Ida arm (4% and 9%) vs the DA arm (1% and 4%) and the DAC arm (0% and 4%; p = 0.034 and p = 0.032, respectively).
- The 5-year overall survival (OS) rate was not improved with FLAG-Ida or DAC vs DA (Figure 1).
- Subgroup analysis suggested that intensification of chemotherapy was beneficial in patients with known MRD (DA vs FLAG-Ida incidence rate ratio [IRR], 0.72; 95% confidence interval [CI], 0.54–0.96; DA vs DAC IRR, 0.83; 95% CI, 0.71–0.98) but not in patients with unknown MRD (DA vs FLAG-Ida IRR, 1.52; 95% CI, 0.92–2.50; DA vs DAC IRR, 1.10; 95% CI, 0.81–1.48).
- A sensitivity analysis in patients with MRD+ showed a 5-year OS rate benefit associated with FLAG-Ida and DAC vs DA (Figure 1).
Figure 1. 5-year OS rate by treatment intensification in the AML18 trial*
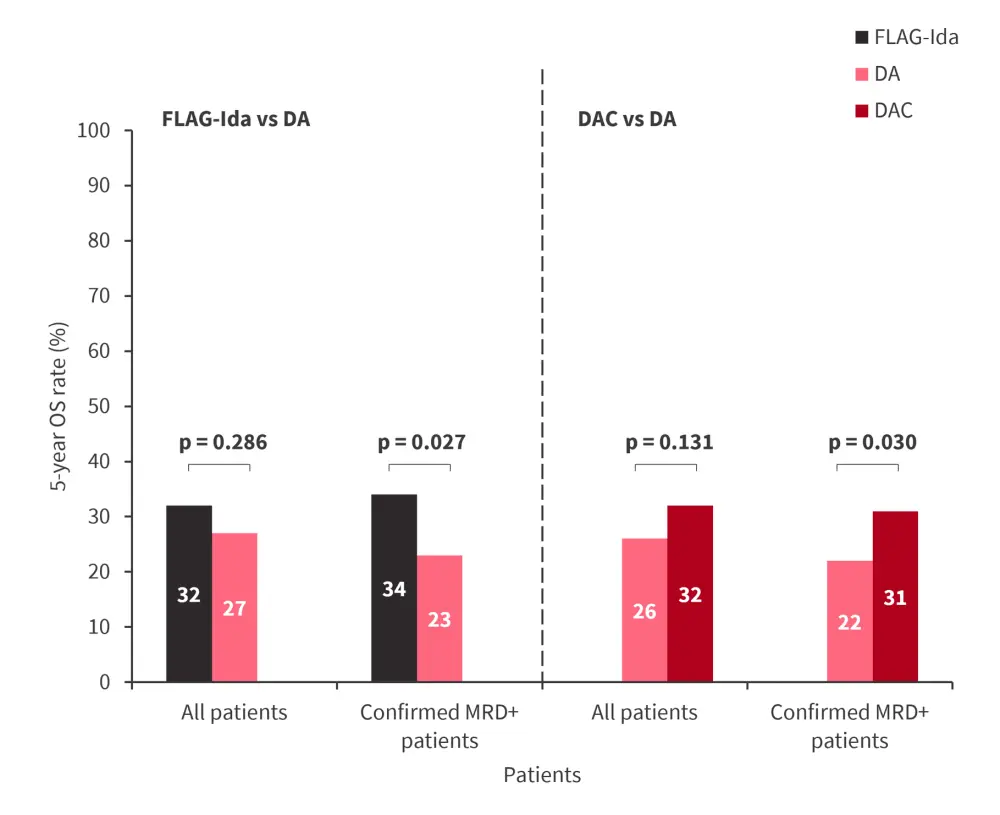
DA, daunorubicin + cytarabine; DAC, DA with cladribine; FLAG-Ida, fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin; MRD, measurable residual disease; OS, overall survival.
*Adapted from Russel.1
- The rate of conversion to MRD negativity in the DA, DAC, and FLAG-Ida arms was 51.3%, 63.3%, and 58.1%, respectively.
- Among patients with confirmed MRD+ status at randomization, the 5-year rate of relapse was higher in the DA arm vs FLAG-Ida arm (70% vs 58%; rate ratio [RR], 0.63; 95% CI, 0.45–0.89; p = 0.008) and vs DAC arm (72% vs 62%; RR, 0.79; 95% CI, 0.66-0.96; p = 0.019).
- In total, 190 patients underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) in first CR (CR1).
- The rate of allo-HSCT in the DA, DAC, and FLAG-Ida arms by randomization was 36%, 42%, and 33%, respectively.
- The survival benefit of the intensification of chemotherapy in MRD+ patients was maintained after adjusting patients for transplant in the DAC arm (Figure 2).
Figure 2. 5-year OS rate by treatment intensification in MRD+ patients censoring patients at allo-HSCT in the AML18 trial*
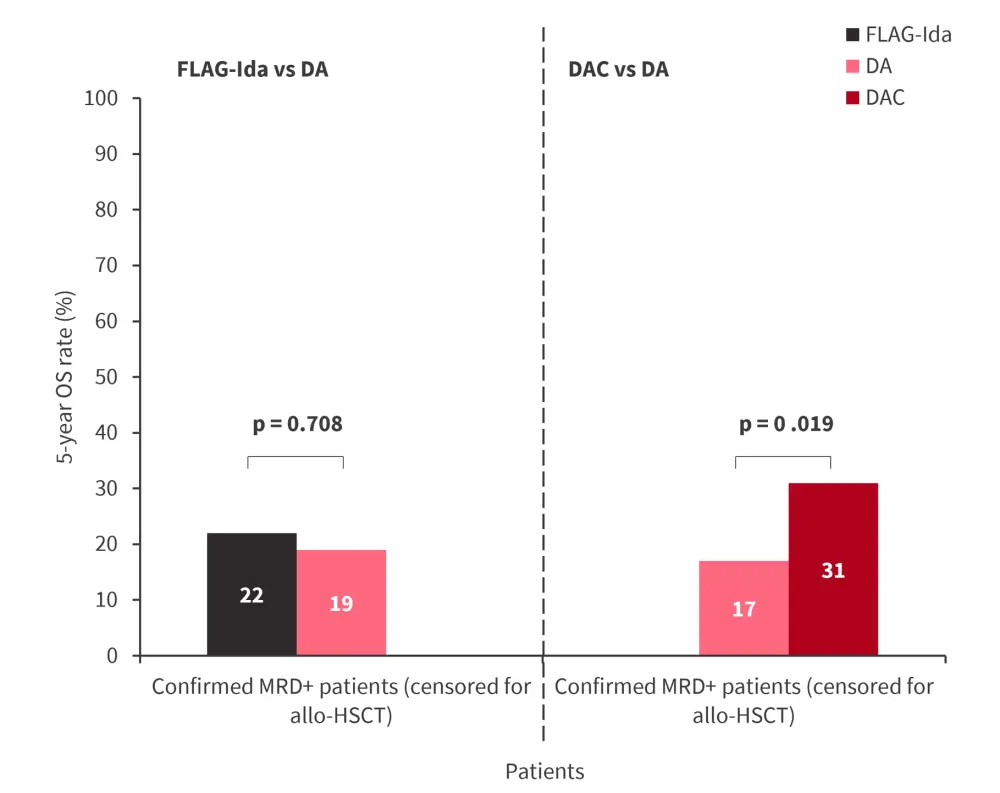
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; DA, daunorubicin + cytarabine; DAC, DA with cladribine; FLAG-Ida, fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin; MRD, measurable residual disease; OS, overall survival.
*Adapted from Russel.1
- The survival benefit of DAC vs DA was also maintained when the analysis was limited to MRD+ patients aged >70 years (DA vs DAC IRR, 0.76; 95% CI, 0.58–0.98).
Presenter’s conclusion
Treatment intensification is associated with a survival benefit in patients with MRD+ status. DAC was better tolerated than FLAG-Ida. The survival benefit of DAC was maintained in patients who did not receive allo-HSCT as well as those who were aged >70 years, while patients treated with FLAG-Ida did not achieve long-term survival benefit.
Interaction between presenting features, mutations, MRD, and induction treatment influence outcomes2
This was a pooled analysis of data from patients with NPM1-mutated AML in the UK AML17 (ISRCTN55675535) and AML19 (ISRCTN78449203) trials. In total, 1357 patients with NPM1 mutated AML were included (AML17, n = 888; AML19, n = 469). The median follow-up was 5.15 years.
Key findings
- Following two courses of chemotherapy:
-
- The 3-year survival rate was 66%, and 93% of patients achieved CR/CRi.
- Patients who were MRD+ had a higher 3-year cumulative incidence of relapse (CIR) rate (65% vs 29%; p < 0.001) and lower 3-year OS rate (40% vs 79%; p < 0.001) compared with MRD negative (MRD−) patients.
- DNMT3A, FLT3-ITD, and WT1 mutations were associated with failure to achieve MRD negativity.
- Multivariable analysis of patients with NPM1-mutated AML showed that increasing age, higher white blood cell count, adverse cytogenetics, DNMT3A, FLT3-internal tandem duplication (ITD), and WT1 mutations were associated with lower OS.
- Patients with adverse cytogenetics or DNMT3A and FLT3-ITD mutations had the lowest OS (3-year OS, 53% for both groups).
- Multivariable analysis of 594 patients who achieved MRD− showed:
- High white blood cell count, DMT3A, IDH1, and WT1 mutations were associated with an increased risk of relapse, while N/KRAS mutations were associated with a decreased risk of relapse.
- Increasing age, DNMT3A, and WT1 mutations were associated with lower OS.
- When compared with other treatments, FLAG-Ida improved the rate of MRD negativity (87% vs 79%; p = 0.009), reduced the relapse rate in patients who were MRD− (16% vs 34%; p < 0.001), and improved the 3-year OS rate (79% vs 63%; hazard ratio [HR], 0.52; p < 0.001).
- The OS benefit associated with FLAG-Ida was greatest in patients with ≥1 feature associated with failure to achieve MRD negativity and relapse in MRD− patients.
Presenter’s conclusion
In patients who achieved MRD−, DNMT3A and WT1 mutations remained associated with poor outcomes, while FLT3-ITD mutations were no longer associated with poor survival outcomes. Survival was improved in patients who received FLAG-Ida, including high-risk patients.
Pretransplant KMT2A-rearranged MRD status associated with poor posttransplant outcomes3
Pre-allo-HSCT cDNA from 66 patients with KMT2A rearranged (KMT2Ar) AML was assessed using RT-qPCR (n = 57) or RT-digital PCR (n = 9). The median age was 42 years, and the median follow-up was 37 months.
Key findings
- The distribution of KMT2Ar fusion partners is shown in Table 2.
Table 2. Distribution of KMT2Ar fusion partners*
|
KMT2Ar fusion partner, % |
All patients (n = 66) |
Pretransplant KMT2Ar MRD status |
|
|---|---|---|---|
|
MRD+ (n = 27) |
MRD− (n = 39) |
||
|
t(9;11)/KMT2A::MLLT3 |
35 |
33 |
36 |
|
t(6;11)/KMT2A::AFDN |
26 |
30 |
23 |
|
t(11;19)/KMT2 A::ELL |
17 |
22 |
13 |
|
t(11;19)/KMT2A::MLLT1 |
5 |
11 |
0 |
|
t(10;11)/KMT2A::MLLT10 |
15 |
4 |
23 |
|
Other |
3 |
0 |
5 |
|
KMT2Ar, KMT2A rearranged; MRD, measurable residual disease; t, translocation. |
|||
- Patients who were KNMT2Ar MRD+ had a lower 2-year relapse-free survival and OS rate, and a higher 2-year CIR rate (Figure 3).
Figure 3. 2-year RFS, OS, and CIR rate by KMT2Ar MRD status*
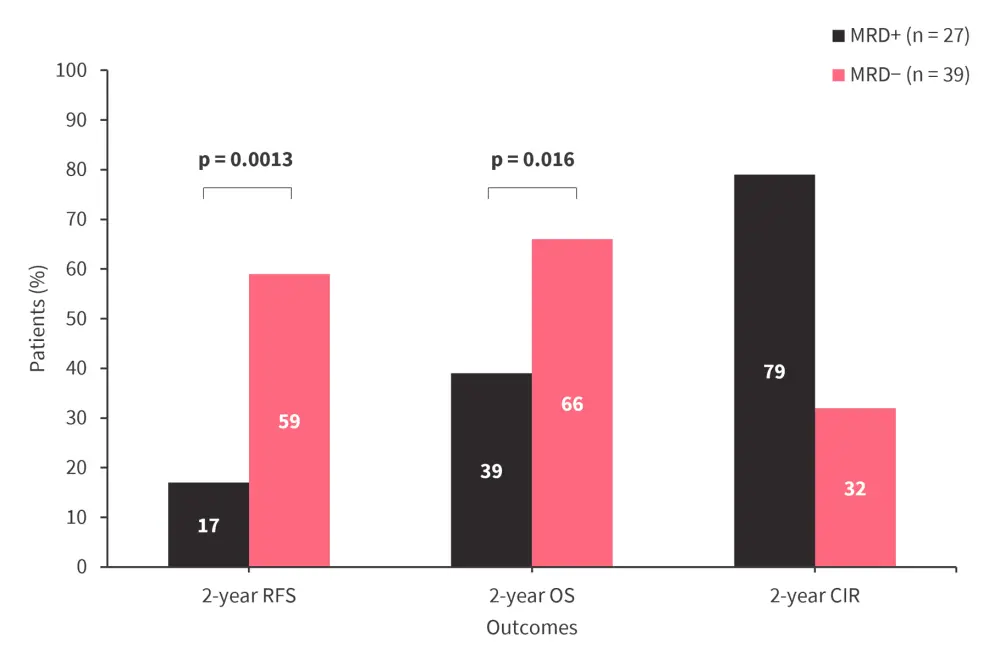
CIR cumulative incidence of relapse; KMT2Ar, KMT2A rearranged; MRD, measurable residual disease; OS, overall survival; RFS, relapse-free survival.
*Data from Loo.3
Presenter’s conclusion
KMT2Ar MRD detected by RT-qPCR/RT-digital PCR pretransplant is associated with worse posttransplant survival outcomes.
Postinduction NPM1 MRD status can be used to select patients for transplantation4
This analysis assessed the impact of MRD status on transplant outcomes in patients with NPM1-mutated AML by combining data from the UK AML17 (ISRCTN55675535) and AML19 (ISRCTN78449203) trials. In the AML17 trial, patients with NPM1-mutated AML in CR1 were selected for transplant based on a validated risk score; whereas, in the AML19 trial, selection was based on MRD status after two courses of chemotherapy, regardless of risk factors. In total, 737 patients were included in this analysis (AML17, n = 348; AML19, n = 389).
Key findings
- In MRD+ patients, allo-HSCT in CR1 was associated with improved OS vs no allo-HSCT (HR, 0.39; 95% CI, 0.24–0.64), while in MRD− patients, allo-HSCT in CR1 did not confer an OS benefit (HR, 0.82; 95% CI, 0.50–1.33).
- When the analysis was limited to patients with NPM1 and FLT3-ITD mutations, allo-HSCT remained beneficial in MRD+ patients (HR, 0.52; 95% CI, 0.29–0.93) but was not associated with a survival benefit in MRD− patients (HR, 0.80; 95% CI, 0.37–1.72).
Presenter’s conclusion
Postinduction MRD status can be used to identify patients with NPM1-mutated AML who are likely to benefit from transplantation in CR1.
Pretransplant IDH2 MRD but not pretransplant IDH1 MRD is associated with poor posttransplant outcomes
Adult patients in CR1 with IDH1- or IDH2-mutated AML undergoing allo-HSCT between 2013 and 2019 were included in these analyses as part of the Pre-MEASURE study. In both analyses, MRD was assessed from peripheral blood samples collected ≤100 days before allo-HSCT using next-generation sequencing.5,6
Key findings
IDH1 cohort5
- This analysis included 148 patients with a median follow-up of 24 months.
- In total, 36% of patients were IDH1 MRD+ prior to allo-HSCT.
- Similar 2-year OS (p = 0.391) and CIR (p = 0.459) rates were observed in patients who were IDH1 MRD+ and MRD−.
- Among 47% of patients with NPM1 and/or FLT3-ITD comutations at baseline, patients who were NPM1 and/or FLT3-ITD MRD+ had a higher 2-year CIR rate vs those who were IDH1 MRD+ only or MRD− (43% vs 11% or 15%; p = 0.011).
- Among the 53% of patients without NPM1 and/or FLT3-ITD comutations at baseline, IDH1 MRD positivity did not impact the 2-year OS (p = 0.218) and CIR (p = 0.253) rates.
IDH2 cohort6
- This analysis included 257 patients with a median follow-up of 25 months.
- 51% of patients were IDH2 MRD+ prior to allo-HSCT.
- Patients who were IDH2 MRD+ had lower 3-year OS (58% vs 83%; p <0.001) and higher 3-year CIR (29% vs 18%; p = 0.03) rates vs patients who were MRD−.
- Among patients without NPM1 and/or FLT3-ITD comutations at baseline, IDH2 MRD was associated with decreased 3-year OS (p = 0.005) and increased 3-year CIR (p = 0.01) rates compared with MRD− patients.
- In the 41% of patients with NPM1 and/or FLT3-ITD comutations at baseline, IDH2 MRD was associated with lower 3-year OS rates (p = 0.007).
- NPM1 and/or FLT3-ITD MRD were the strongest prognostic factors for posttransplant outcomes (Table 3).
Table 3. Factors associated with posttransplant outcomes in the IDH2 cohort from the Pre-MEASURE study*
|
OS |
HR (95% CI) |
p-value |
|---|---|---|
|
NPM1/FLT3-ITD MRD+ |
10 (3.2–31) |
<0.001 |
|
IDH2 MRD+ |
4.4 (1.4–13.7) |
0.01 |
|
Relapse |
HR (95% CI) |
p-value |
|
NPM1/FLT3-ITD MRD+ |
21.0 (7.0–63) |
<0.001 |
|
Age, >60 years |
5.0 (1.4–18) |
0.02 |
|
Age, every 1 year above 40 |
0.95 (0.9–0.97) |
<0.001 |
|
IDH1 baseline, positive |
4.7 (1.5–14.3) |
0.007 |
|
Antithymocyte globulin, yes |
2.9 (1.1–7.3) |
0.03 |
|
Sex, male |
0.3 (0.1–0.9) |
0.03 |
|
CI, confidence interval; HR, hazard ratio; ITD, internal tandem duplication; MRD+, measurable residual disease positive; OS, overall survival. *Adapted from Gui.6 |
||
Presenter’s conclusion
Pre-allo-HSCT IDH1 MRD status was not associated with post-allo-HSCT outcomes.5 However, pre-allo-HSCT NMP1 and/or FLT3-ITD MRD positivity was associated with an increased risk of relapse in patients with IDH1-mutated and NMP1 and/or FLT3-ITD-mutated AML.5 Conversely, pre-allo-HSCT IDH2 MRD positivity was predictive of inferior post-allo-HSCT outcomes.6 In patients with IDH2 and NMP1 and/or FLT3-ITD mutations at baseline, NMP1 and/or FLT3-ITD MRD status was a superior prognostic marker than IDH2 MRD status.6
Your opinion matters
Do you intend to implement next generation sequencing for measurable residual disease monitoring in AML patients?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




