All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Impact of MRD status on outcomes in patients with AML treated with different treatment regimens
Morphologic response assessment in patients with acute myeloid leukemia (AML) may lack sensitivity and a small number of leukemic cells may remain after treatment in a patient who is morphologically in complete remission (CR).1 Highly sensitive tests can detect 0.1–0.01% AML blasts in patients who are in CR, referred to as measurable residual disease (MRD).1 The detection of MRD has been associated with inferior survival outcomes and an increased risk of relapse.1 However, most studies on the prognostic value of MRD have focused on patients treated with intensive chemotherapy.1 The detection of MRD pre-allogeneic hematopoietic stem cell transplantation (allo-HSCT) may also help to identify patients who are at an increased risk of relapse post allo-HSCT.2
Bazinet et al.1 recently published a study in Blood Advances assessing the impact of MRD on outcomes in patients with AML treated with either higher- or lower-intensity regimens. The pre‑MEASURE study by Dillon et al.2 published in JAMA evaluated the impact of pretransplant MRD on allo-HSCT outcomes. The AML Hub has previously reported the pre-MEASURE study, focusing on the prognostic significance of pretransplant MRD testing. We are pleased to present the key findings from these two studies here.
MRD assessment with different treatment intensities1
Study design and patient characteristics
This was a retrospective cohort study involving 635 patients with newly diagnosed AML who achieved either CR, CR with incomplete hematologic recovery (CRi), or morphological leukemia-free state (MLFS) and had availability of MRD data. Eligible patients were either treated with intensive chemotherapy regimens with intermediate- to high-dose cytarabine plus an anthracycline (IA cohort) or low-intensity therapy with low-dose cytarabine or hypomethylating agent backbone plus venetoclax (LOW + VEN cohort) at the MD Anderson Cancer Center, US, between February 2010 and October 2021. Table 1 shows the baseline characteristics.
Table 1. Patient characteristics*
|
CR, complete remission; CRi, CR with incomplete hematologic recovery; ELN, European LeukemiaNet; IA, idarubicin plus intermediate to high-dose cytarabine; LOW + VEN, low-intensity backbone plus venetoclax; MLFS, morphologic leukemia-free state; MRD, measurable residual disease. |
|||
|
Characteristic, % (unless otherwise stated) |
IA cohort |
LOW + VEN cohort |
p value |
|---|---|---|---|
|
Median age (range), years |
52.8 (17.1–77.7) |
71.6 (25.6–89.1) |
<0.001 |
|
Age ≥60 years |
15.1 |
95.6 |
<0.001 |
|
Sex |
|
|
0.004 |
|
Male |
46.2 |
58.0 |
— |
|
Female |
53.8 |
42.0 |
— |
|
Best response |
|
|
<0.001 |
|
CR |
89.4 |
76.0 |
— |
|
CRi |
8.8 |
16.8 |
— |
|
MLFS |
1.8 |
7.2 |
— |
|
MRD status |
|
|
0.004 |
|
Negative |
71.4 |
60.4 |
— |
|
Positive |
28.6 |
39.6 |
— |
|
ELN 2017 risk |
|
|
<0.001 |
|
Favorable |
27.5 |
22.0 |
— |
|
Intermediate |
36.4 |
20.0 |
— |
|
Adverse |
33.5 |
57.6 |
— |
|
Cytogenetics |
|
|
— |
|
Diploid |
52.2 |
42.4 |
0.016 |
|
Other intermediate |
16.1 |
15.2 |
0.760 |
|
11q23 |
6.0 |
4.4 |
0.389 |
|
t(6;9) |
1.6 |
0.8 |
0.490 |
|
inv(3) |
1.3 |
2.0 |
0.488 |
|
−5/5q− |
7.3 |
19.2 |
<0.001 |
|
−7/7q− |
7.3 |
16.8 |
<0.001 |
|
−17/17p− |
5.5 |
12.4 |
0.002 |
|
Complex |
16.4 |
29.2 |
<0.001 |
Key findings
Responses
The median time from the initiation of therapy to best morphologic response was 32 days (interquartile range, 27–38 days) in the IA cohort and 39 days (interquartile range, 30–63 days) in the LOW + VEN cohort. More patients received allo-HSCT in the IA versus LOW + VEN cohort (50.1% vs 24.8%). Figure 1 and Figure 2 show the distribution of CR responses and MRD negativity by 2017 European LeukemiaNet (ELN) adverse risk, respectively.
Figure 1. CR responses by ELN risk category*
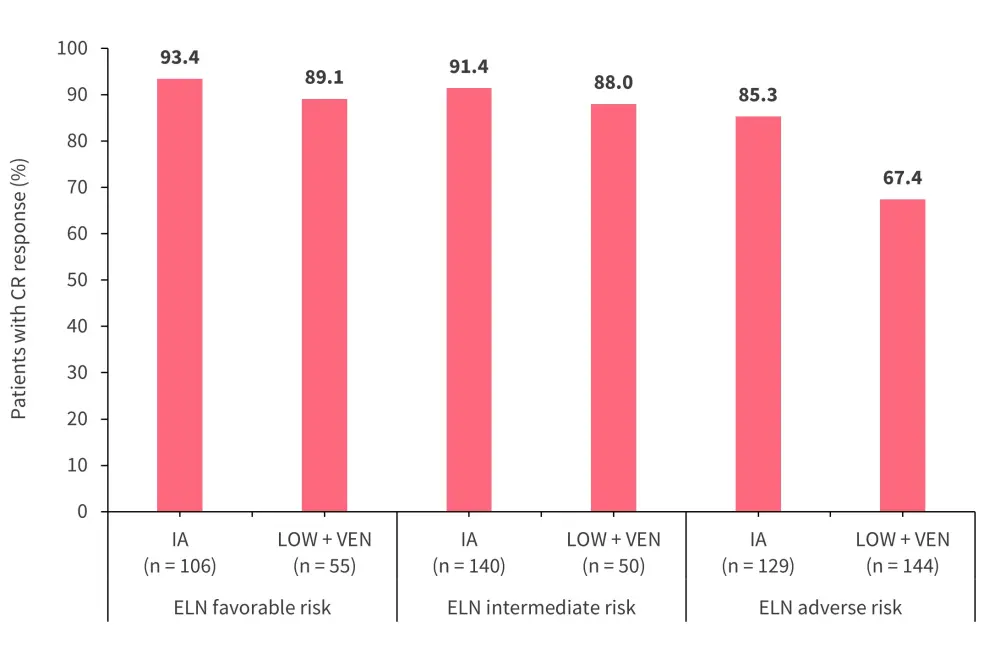
CR, complete remission; ELN, European LeukemiaNet; IA, idarubicin plus intermediate to high-dose cytarabine; LOW + VEN, low-intensity backbone plus venetoclax.
*Adapted from Bazinet, et al.1
Figure 2. MRD negativity rates by ELN risk category*
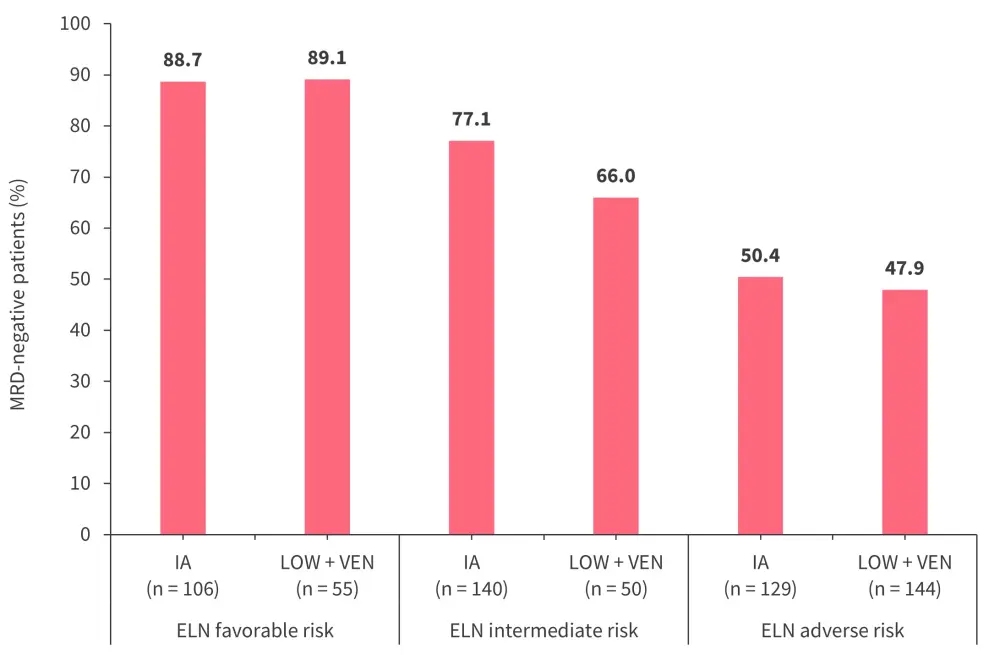
ELN, European LeukemiaNet; IA, idarubicin plus intermediate to high-dose cytarabine; LOW + VEN, low-intensity backbone plus venetoclax; MRD, measurable residual disease.
*Adapted from Bazinet, et al.1
Survival outcomes and cumulative incidence of relapse
Patients were grouped by treatment received and MRD status as shown in Table 2. The median follow-up was 38.9 months.
- Among patients in the IA cohort, MRD negativity was associated with a longer median overall survival (OS) compared with those in the LOW + VEN cohort (p = 0.001).
- However, the cumulative incidences of relapse (CIR) rates were similar between the two groups (p = 0.300; Table 2).
- Similarly, patients who were MRD positive in the IA cohort had a longer median OS compared with those in the LOW + VEN cohort (p = 0.018).
- The 2-year CIR rates among patients who were MRD positive in both cohorts were comparable (p = 0.624).
|
CIR, cumulative incidence of relapse; CITRM, cumulative incidence of treatment-related mortality; IA, idarubicin plus intermediate to high-dose cytarabine; LOW + VEN, low-intensity backbone plus venetoclax; MRD, measurable residual disease; OS, overall survival. |
||||
|
Outcome |
MRD negative |
MRD positive |
||
|---|---|---|---|---|
|
IA |
LOW + VEN |
IA |
LOW + VEN |
|
|
Median OS, months |
50.2 |
18.2 |
13.6 |
8.1 |
|
2-year CIR, % |
41.1 |
33.5 |
64.2 |
59.9 |
|
2-year CITRM, % |
7.8 |
29.5 |
9.4 |
30.4 |
Table 2. Outcomes by MRD and treatment status*
Univariate analysis revealed:
- Younger age, higher-intensity therapy, achievement of CR, MRD negativity, lower ELN risk, and non-adverse karyotype were associated with improved OS, while achievement of CR, MRD negativity, lower ELN risk, and non-adverse cytogenetics were associated with lower CIR rates.
Multivariable analysis showed the following:
- Age (p = 0.025), best response (CR vs CRi, p = 0.033; CR vs MLFS, p < 0.001), MRD status (p = 0.006), and ELN risk (favorable vs adverse, p = 0.001) were significantly associated with OS.
- Best response (CR vs CRi, p = 0.001), MRD status (p = 0.001), and ELN risk (favorable vs intermediate, p = 0.003; favorable vs adverse, p < 0.001) were significantly associated with CIR.
Among patients with ELN favorable risk, those who achieved MRD negativity had longer OS when treated with IA compared with those treated with LOW + VEN (80.9 months vs 30.8 months; p = 0.012). Survival analysis without censoring patients who received allo-HSCT showed that patients treated with IA had significantly improved OS. This was likely due to the higher proportion of patients in this group receiving allo-HSCT.
MRD pre-allo-HSCT2
Study design and patient characteristics
This was a retrospective cohort study with 1,075 patients aged ≥18 years with AML with FLT3, NPM1, IDH2, and/or KIT mutations who received allo-HSCT in first CR. Patient data were obtained from 111 centers using the Center for International Blood and Marrow Transplant Research (CIBMTR) database. The discovery cohort comprised 454 patients who underwent allo-HSCT between March 2013 and December 2017 and the validation cohort comprised 621 patients who underwent allo-HSCT between January 2018 and February 2019. Patient characteristics are detailed in our previous article. Patients from both cohorts with NPM1 and/or FLT3 internal tandem duplication (FLT3-ITD) mutations (n = 822) are the focus of this analysis.
The primary outcomes included OS and CIR and the secondary outcomes were relapse-free survival (RFS) and non-relapse mortality (NRM).
Key findings
- The 3-year relapse rates were similar between the discovery and validation cohorts (29% vs 28%).
- Similarly, the 3-year OS rates were comparable between the two cohorts (61% vs 61%).
- Secondary AML, ELN adverse or intermediate risk, and reduced intensity conditioning were associated with increased relapse rates.
- In the discovery cohort, 28.9% of patients had FLT3, NPM1, IDH1, IDH2, or KIT mutations detected in pretransplant remission blood by next-generation sequencing-MRD, while in the validation cohort, 30.3% of patients had a mutation detected in the same set of genes.
- In the discovery cohort, the detection of mutations pretransplant was associated with increased relapse, lower RFS, and decreased OS, but not NRM.
- In the univariate analysis, the presence of FLT3-ITD or NPM1 mutations was associated with increased relapse in the discovery cohort.
Overall survival and cumulative incidence of relapse
In the discovery cohort:
- Of the 371 patients with NPM1 and/or FLT3-ITD mutations, 64 patients with persistent mutations had higher 3-year post-transplant relapse rates (59% vs 24%; hazard ratio [HR], 3.71; p < 0.001) and lower 3-year OS rates (34% vs 66%; HR, 2.60; p < 0.001) compared with patients who did not have persistent mutations.
In the validation cohort:
- Of the 451 patients with NPM1 and/or FLT3-ITD mutations, 78 patients with persistent mutations had higher 3-year post-transplant relapse rates (68% vs 21%; HR, 4.32; p < 0.001) and lower 3-year OS rates (39% vs 63%; HR, 2.43; p < 0.001) compared with patients without persistent mutations.
Relapse-free survival and non-relapse mortality
In the discovery cohort:
- The presence of persistent FLT3-ITD and/or NPM1 mutations were associated with lower rates of RFS (27% vs 59%) compared with patients who did not have persistent FLT3-ITD and/or NPM1 mutations. The NRM rates were similar between the two groups.
In the validation cohort:
- Patients with persistent FLT3-ITD and/or NPM1 mutations had lower rates of RFS (19% vs 59%) when compared with patients who did not have persistent FLT3-ITD and/or NPM1 mutations. The NRM rates were also similar between the two groups.
Molecular subgroups
Among patients who had FLT3-ITD or NPM1 mutations at baseline, the presence of residual FLT3-ITD or NPM1 mutations were associated with higher rates of relapse, lower rates of RFS, and lower OS when compared with patients without residual mutations (Figure 3).
Figure 3. 3-year relapse rates, RFS rates, and OS rates*
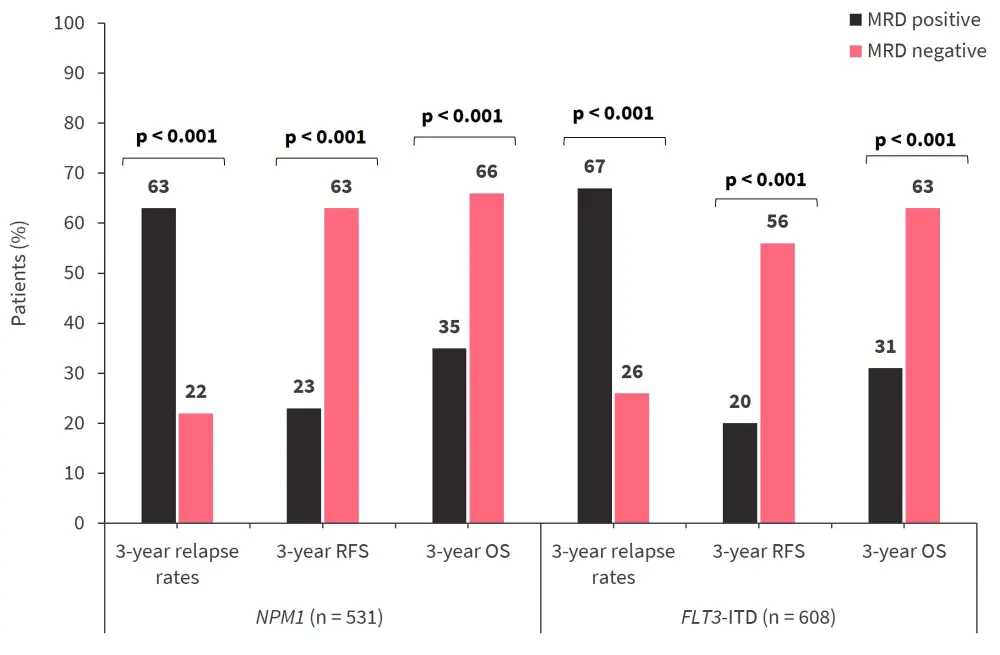
ITD, internal tandem duplication; MRD, measurable residual disease; OS, overall survival; RFS, relapse-free survival.
*Data from Dillon, et al.2
Transplant intensity
While MRD positivity was associated with higher rates of relapse and worse OS, this was partially mitigated in patients aged <60 years by high-intensity myeloablative conditioning (3-year relapse rate, 53% vs 78%; HR, 1.97; p = 0.04). Multivariable analyses revealed that MRD detected by next-generation sequencing was associated with relapse and OS, and reduced-intensity conditioning without melphalan was also associated with higher rates of relapse.
Conclusion
The study by Bazinet et al.1 shows the benefit of achieving MRD negativity in patients treated with IA or LOW + VEN treatments. The findings indicate that disease control is similar between the two treatment types once the best response is achieved. In the pre-MEASURE study, persistent FLT3-ITD or NPM1 mutations pretransplant were associated with inferior outcomes posttransplant, indicating that MRD detection may improve prognostication. Routine DNA sequencing to detect MRD may improve outcomes for patients with AML. Both studies are limited by their retrospective nature, and results need to be confirmed in prospective studies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


