All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Phase I/II study of the FLT3 inhibitor pexidartinib in patients with relapsed/refractory AML with FLT3-ITD mutations
Featured:
FMS-like tyrosine kinase 3 (FLT3) mutations, such as internal tandem duplication (ITD) and point mutations in the FLT3 tyrosine kinase domain (TKD), are common in acute myeloid leukemia (AML).
Tyrosine kinase inhibitors (TKIs) of FLT3 that demonstrate activity in patients with FLT3 mutations include the following:
- Midostaurin, a multitargeted inhibitor, approved for the treatment of patients with newly diagnosed FLT3-mutant AML
- Quizartinib and gilteritinib, next-generation FLT3 inhibitors, showing high response rates as monotherapy in relapsed/refractory (R/R) patients with FLT3 mutations
- Quizartinib and gilteritinib both confer a survival benefit, compared with salvage chemotherapy, in patients with R/R FLT3-mutant AML
- Gilteritinib is approved for the treatment of patients with R/R FLT3-mutant AML
Unfortunately, because of the development of therapeutic resistance, survival in patients with R/R FLT3-mutant AML remains poor. Currently, there are limited options for the treatment of patients with FLT3 TKI resistance, commonly due to mutations in the FLT3 TKD at the kinase gatekeeper residue F691 (F691L mutation), which interfere with the binding capacity of TKIs.
Pexidartinib is a small-molecule kinase inhibitor with selective activity against colony-stimulating factor 1 receptor (CSF1R), proto-oncogene receptor tyrosine kinase, and FLT3-ITD. Pexidartinib is able to maintain binding to FLT3 with a F691L mutation, and preclinical studies have shown that pexidartinib is active in cells expressing FLT3-ITD/F691L mutations. In this phase I/II study, published in Blood Advances, Catherine Smith and colleagues evaluated the safety and efficacy of pexidartinib in patients with R/R AML with FLT3-ITD mutations.
Study design and patient characteristics
This open-label, phase I/II trial enrolled 90 patients with R/R FLT3-ITD+ AML and consisted of a dose-escalation part (Part 1; n = 34) followed by a dose-expansion cohort (Part 2; n = 56). The primary objective of the dose-escalation part was to determine the maximum tolerated dose (MTD) and recommend a phase 2 dose (RP2D) of pexidartinib to treat patients in a subsequent dose-expansion cohort. The Part 2 objective was to determine the overall response rate at the RP2D:
- Primary efficacy endpoint: Rate of composite complete remission (CRc), including complete remission (CR), CR with incomplete recovery (CRi), and CR with incomplete platelet recovery (CRp), plus the percentage of patients who were successfully bridged to transplantation
- Secondary endpoints: Partial remission (PR), duration of remission, overall survival (OS), and progression-free survival (PFS)
Pexidartinib was given orally twice daily from Day 1 to Day 28 of each 28-day cycle. The design of the study is reported in Figure 1. Baseline characteristics of patients enrolled in the study are shown in Table 1.
Figure 1. Study design1
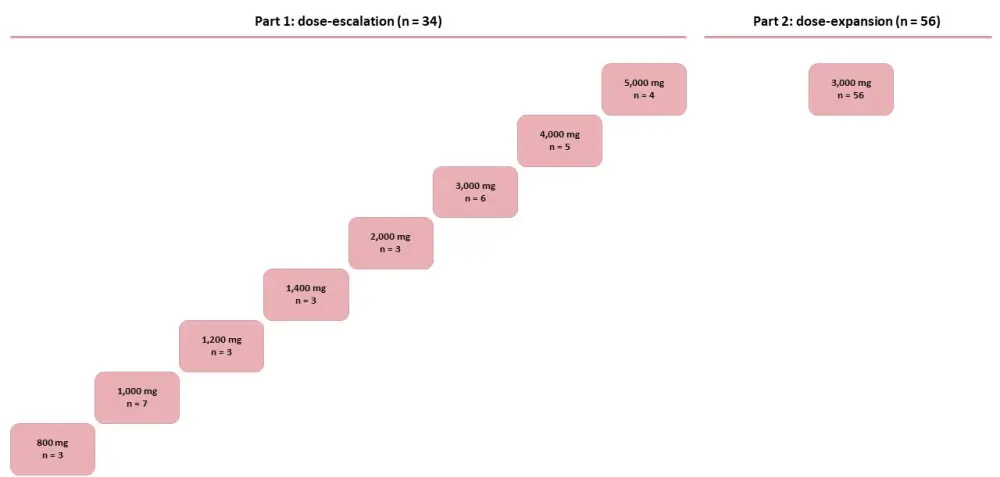
Table 1. Patient characteristics
|
AML, acute myeloid leukemia; ECOG, Eastern Cooperative Oncology Group; MDS, myelodysplastic syndrome |
||
|
Characteristic |
Part 1 (n = 34) |
Part 2 (n = 56) |
|---|---|---|
|
Age, years (range) |
60.6 (24–82) |
55.9 (22–83) |
|
Sex, n (%) Male Female |
13 (38) 21 (62) |
33 (59) 23 (41) |
|
ECOG performance status, n (%) Fully active (0) Restricted (1) Ambulatory (2) |
6 (18) 17 (50) 11 (32) |
9 (16) 33 (59) 14 (25) |
|
AML category at diagnosis, n (%) De novo Secondary to MDS Secondary to chemotherapy for another cancer Unknown |
26 (76) 4 (12) 2 (6)
2 (6) |
44 (79) 2 (4) 4 (7)
6 (11) |
|
FLT3-ITD+ at diagnosis, n (%) Yes No Unknown |
30 (88) 2 (6) 2 (6) |
45 (80) 4 (7) 7 (13) |
|
Prior FLT3 inhibitor therapy, n (%) |
14 (41) |
12 (21) |
|
Prior allogeneic transplantation, n (%) Yes No Unknown |
7 (21) 27 (79) 0 |
21 (38) 34 (61) 1 (2) |
|
Prior AML therapies, median (range) |
4 (2–12) |
4 (1–10) |
Results
Data cutoff date was January 20, 2015.
Safety
In the dose-escalation part, eight different doses of pexidartinib were investigated (Figure 1), and no dose-limiting toxicities related to study treatment were observed.
The most frequent treatment-emergent adverse events (TEAEs) occurring in ≥ 20% of patients are reported in Table 2. Twelve patients (13%) died during the study (Part 1, n = 3; Part 2, n = 9) because of sepsis (n = 5), pneumonia (n = 2), pneumonia aspiration (n = 1), respiratory failure (n = 1), cardiac arrest (n = 1), cytokine release syndrome (differentiation syndrome; n = 1), and cerebral hemorrhage (n = 1). Only the death due Grade 5 cytokine release syndrome, which occurred in Part 2, was considered related to the treatment.
Table 2. TEAEs occurring in ≥ 20% of patients1
|
TEAE, treatment-emergent adverse event |
|||
|
TEAE, n (%) |
Grade 1–2 |
Grade ≥ 3 |
All grades |
|---|---|---|---|
|
Diarrhea |
44 (49) |
1 (1) |
45 (50) |
|
Fatigue |
33 (37) |
9 (10) |
42 (47) |
|
Nausea |
40 (44) |
1 (1) |
41 (46) |
|
Febrile neutropenia |
0 (0) |
38 (42) |
38 (42) |
|
Decreased appetite |
31 (34) |
2 (2) |
33 (37) |
|
Vomiting |
32 (36) |
1 (1) |
33 (37) |
|
Cough |
26 (29) |
0 (0) |
26 (29) |
|
Anemia |
9 (10) |
15 (17) |
24 (27) |
|
Hypokalemia |
16 (18) |
6 (7) |
22 (24) |
|
Aspartate aminotransferase increased |
14 (16) |
5 (6) |
19 (21) |
|
Dyspnea |
15 (17) |
3 (3) |
18 (20) |
Pharmacokinetic data indicated that at pexidartinib doses ˃ 3,000 mg, plasma exposure levels did not increase. Therefore, the dose of 3,000 mg was chosen as the R2PD and used in the dose expansion part of the study.
Efficacy
For all treated patients (N = 90), the overall response rate (≥ PR) was 21% (n = 19), and the overall composite CRc ( CRc consisting of CR, CRi, and CRp) rate was 11% (n = 10). The best responses observed in Part 1 and Part 2 of the study are reported in Figure 2.
Six patients underwent hematopoietic stem cell transplantation (HSCT; three after response: PR, n = 1; CRi, n = 1; CRp, n = 1):
- Three received pexidartinib maintenance after HSCT
- Two discontinued maintenance (one relapsed 6 months after HSCT, and one relapsed 2 years after HSCT)
- One patient was still on treatment without relapse at the cutoff date
- Of the remaining three
- Two relapsed 100 days post-HSCT
- One was lost to follow-up
Figure 2. Response rates in A Part 1 and B Part 2 of the study1
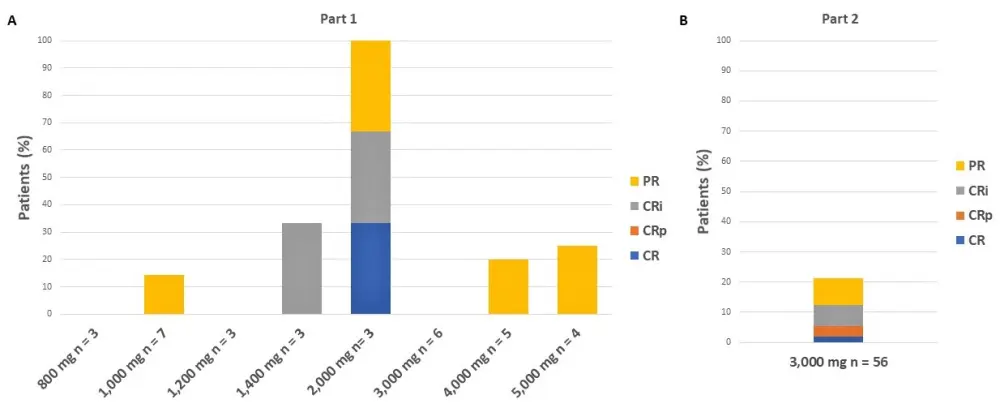
The median duration of response, PFS, and OS stratified for response and pexidartinib dose are reported in Table 3. In responders (≥ PR) vs non-responders, the median PFS was extended by 98 days and the median OS by 171 days. No significant differences in PFS and OS were found between patients treated at doses < 3,000 mg and patients treated at doses ≥ 3,000 mg.
Table 3. Secondary endpoints1
|
CR, complete remission; CRc, composite CR; CRi, CR with incomplete recovery; CRp, CR with incomplete platelet recovery; OS, overall survival; PFS, progression-free survival; PR, partial remission |
||||
|
|
Median duration of response |
Median PFS |
Median OS |
Median time to best response |
|---|---|---|---|---|
|
Part 1, days |
74 |
57 |
91 |
30 |
|
Part 2, days |
76 |
48 |
112 |
30 |
|
Responders (≥ PR) vs non-responders, days |
— |
133 vs 35 |
250 vs 79 |
— |
|
Complete responders (CRi, CRp, or CR), days |
— |
— |
265 |
— |
|
CRc (n = 9), days |
212 |
— |
— |
— |
|
Pexidartinib < 3,000 vs ≥ 3,000 mg |
— |
57 vs 52 |
92 vs 96 |
— |
In patients pretreated with TKI, the response rate was lower than in FLT3 TKI-naïve patients: 14.8% (4/27 patients; CR, n = 1; PR, n = 3) vs 23.8% (15/63 patients; CR, n = 1; CRi, n = 6; CRp, n = 2; PR, n = 6), respectively.
The potential clinical activity of pexidartinib in patients with FLT3 F691L mutations was evaluated in four patients with F691L mutations at the time of drug initiation. Of these patients, two were treated at a dose of 1,000 mg per day: one at 1,200 mg and one at 2,000 mg. Only the patient treated at the dose of 2,000 mg achieved a CRi.
Conclusion
This study shows that pexidartinib is well tolerated in heavily pretreated patients with R/R FLT3-ITD+ AML. Survival benefits were observed in responders (≥ PR) vs non-responders, with a median PFS extended by 98 days and median OS by 171 days.
Further studies are required to determine the clinical activity of pexidartinib in patients with FLT3 F691L mutations because of the small number of patients with F691L mutations in this study.
Expert Opinion
This phase I/II trial of the novel oral FLT3 inhibitor, pexidaritinib, in patients with relapsed/refractory FLT3 ITD mutant AML was disappointing with much lower response rates (21%) than that achieved with other second generation FLT3 inhibitors (gilteritinib, quizartinib) in similar patients. This may have been due to decreased potency of this agent with doses of ≥3000 mg required for maximal FLT3 inhibitor. Nevertheless pexidaritinib was overall well tolerated and its potential activity against the FLT3 F691L gatekeeper mutation conferring resistance to all other known inhibitors suggests it may be useful for patients whose disease has progressed following other FLT3 inhibitor therapy such as gilteritinib.
 Eunice Wang
Eunice WangReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

