All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Oral decitabine and cedazuridine + venetoclax for the treatment of older patients with newly diagnosed AML
Older patients aged ≥60 years with acute myeloid leukemia (AML) are often not eligible for standard chemotherapy regimens due to adverse risk disease features.1 However, for this patient population, low-intensity regimens compare favorably with intensive chemotherapy.1 One promising low-intensity regimen is the oral decitabine and cedazuridine (ASTX727) + venetoclax.
Recently, Bazinet et al.1 published results from a study (NCT04746235) evaluating the safety and efficacy of ASTX727 + venetoclax in older patients with newly diagnosed AML (ND AML) or with relapsed/refractory AML (R/R AML) in the Lancet Hematology. Here, we summarrize the key results.
Study design1
- This is an ongoing single-center, phase II study.
- Treatment cycles were 28 days long for up to 24 cycles
- ASTX727 (cedazuridine 100 mg and decitabine 35 mg) was administered orally on Days 1–5
- Venetoclax 400 mg was administered orally from Days 21–28
- The primary endpoint was the overall response rate.
- Secondary endpoints were overall survival, relapse-free survival, duration of response, and safety.
Key findings1
- A total of 62 patients were included, 49 with ND AML and 13 with R/R AML.
- The median follow-up period was 18.3 months.
- The response rates for both patient groups are shown in Figure 1.
Figure 1. Response rates in patients with ND AML or R/R AML treated with ASTX727 + venetoclax*
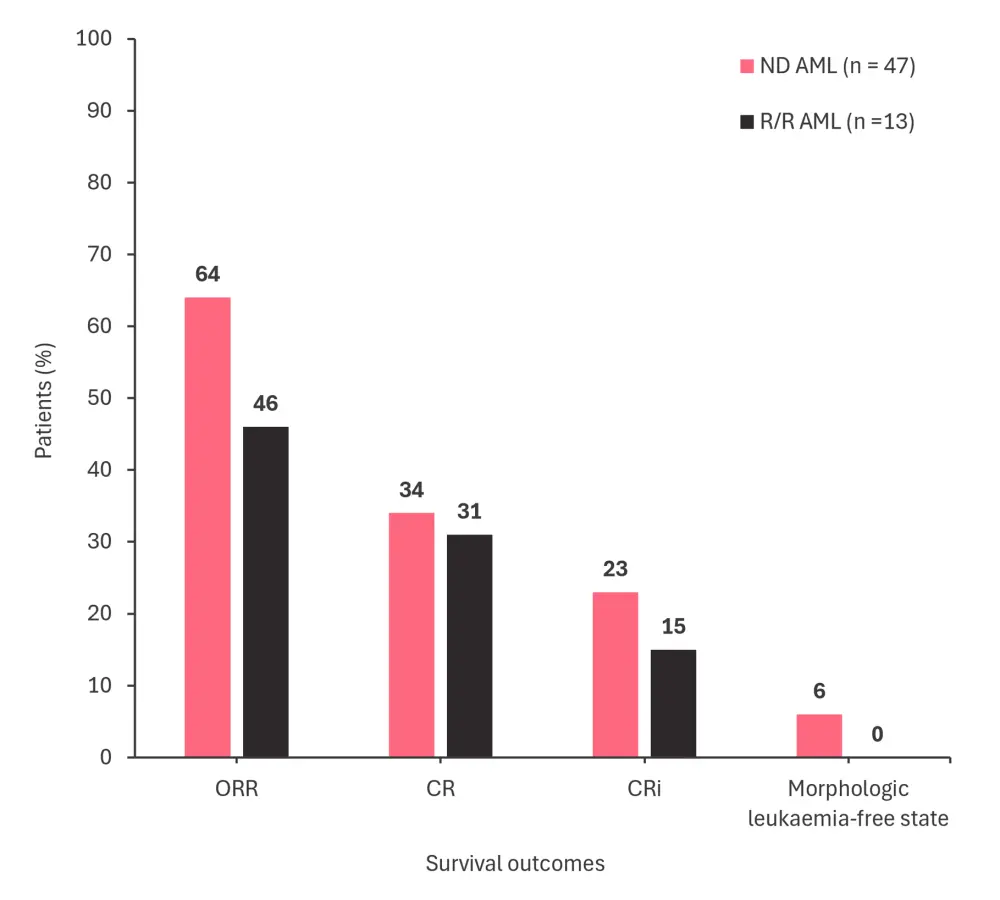
CR, complete remission; CRi, CR with incomplete count recovery; ND AML, newly diagnosed acute myeloid leukemia; ORR, overall response rate; R/R AML, relapsed/refractory acute myeloid leukemia.
*Adapted from Bazinet, et al.1
- Of the evaluable patients, negative measurable residual disease was achieved by 44% of patients with ND AML vs 20% with R/R AML.
- The secondary survival endpoints are shown in Table 1.
Table 1. Secondary survival endpoints for patients with ND AML or R/R AML treated with ASTX727 + venetoclax*
|
Secondary survival endpoints |
ND AML (n = 47) |
R/R AML (n = 13) |
|---|---|---|
|
Median OS, months |
11.5 |
7.2 |
|
1-year OS, % |
49.0 |
18.0 |
|
2-year OS, % |
18.0 |
18.0 |
|
Median RFS, months |
12.0 |
4.6 |
|
1-year RFS, % |
47.0 |
17.0 |
|
Median DOR, months |
13.2 |
5.4 |
|
1-year DOR, % |
51.0 |
25.0 |
|
AML, acute myeloid leukemia; DOR, duration of response; ND, newly diagnosed; OS, overall survival; RFS, relapse free survival; R/R, relapsed/refractory. *Data from Bazinet, et al.1 |
||
- Overall, 82% of patients experienced any-grade treatment-emergent adverse events (TEAEs). The most common were:
- constipation (23%);
- oral mucositis (23%); and
- febrile neutropenia (18%).
- Overall, 63% of patients experienced a Grade ≥3 TEAEs. The most common were:
- febrile neutropenia (18%);
- pneumonia (13%); and
- Respiratory failure (8%).
- There were four fatal TEAEs.
|
Key learnings |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




