All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Venetoclax plus decitabine vs intensive chemotherapy for young patients with ND AML
Venetoclax plus hypomethylating agent treatment is typically recommended for older/unfit patients with acute myeloid leukemia (AML).1 A previous phase II trial (NCT04752527) suggested that venetoclax plus decitabine (Ven-Dec) may be beneficial in newly diagnosed (ND) young adult patients with European LeukemiaNet (ELN) 2017 adverse risk AML.1 However, the potential benefit of this combination in ND young adults with ELN 2017 favorable or intermediate risk AML who are fit for intensive chemotherapy is not well characterized.1
During the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, Lu1 presented interim results from a phase IIb trial comparing the safety and efficacy of venetoclax plus decitabine vs intensive chemotherapy as induction therapy in ND patients with AML. Below, we summarize the key findings.
Study design1
- This was a multicenter, randomized, phase IIb trial (NCT05177731).
- The primary endpoint was the rate of composite complete remission (CRc).
- Secondary endpoints included rate of measurable residual disease (defined as <1×10−3 by flow cytometry), safety, event-free survival, and overall survival.
Key findings1
- Overall, 134 patients with ND AML receiving either Ven-Dec (n = 67) or idarubicin and cytarabine (IA-12; n = 67) were included; median age was 45 years and 40 years, respectively.
Response
- CRc rate was significantly higher with Ven-Dec vs IA-12 following the second induction cycle (Figure 1).
Figure 1. Response after A 1st and B 2nd induction cycle by treatment type*
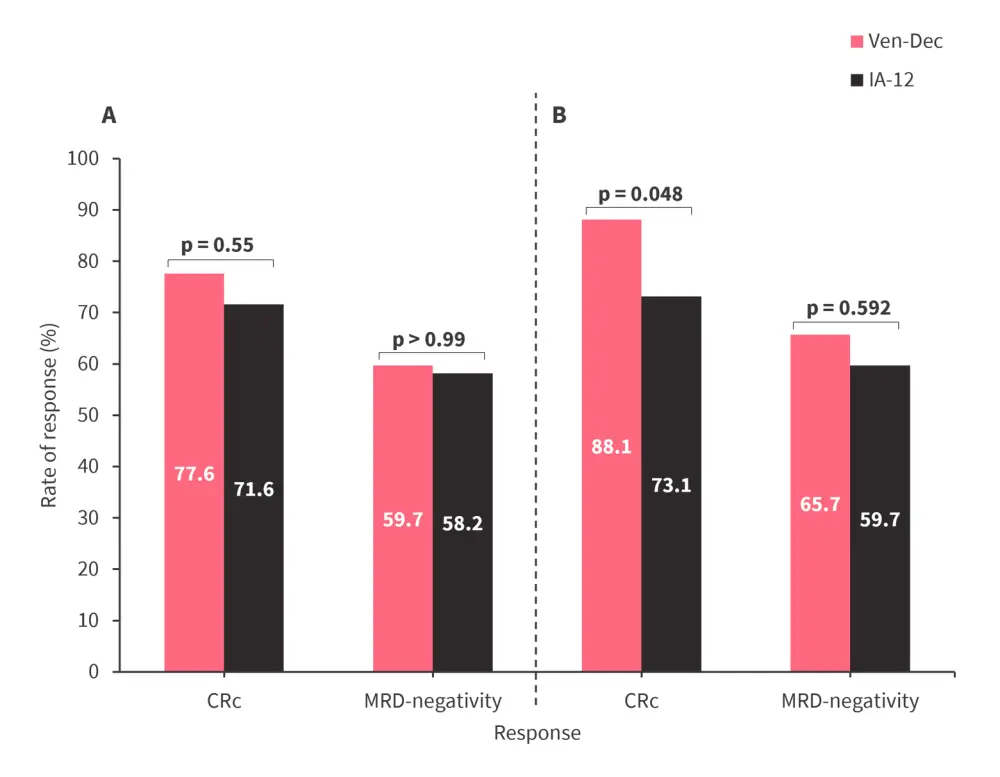
CRc, composite complete remission; IA-12, idarubicin and cytarabine; MRD, measurable residual disease; Ven-Dec, venetoclax and decitabine.
*Adapted from Lu.1
- When analyzed by ELN 2017 risk group, the CRc rate after the second induction cycle was significantly higher with Ven-Dec vs IA-12 for patients in the intermediate and adverse risk groups (Figure 2).
Figure 2. A CRc and B MRD-negativity rate after 2nd induction cycle by ELN 2017 risk group*
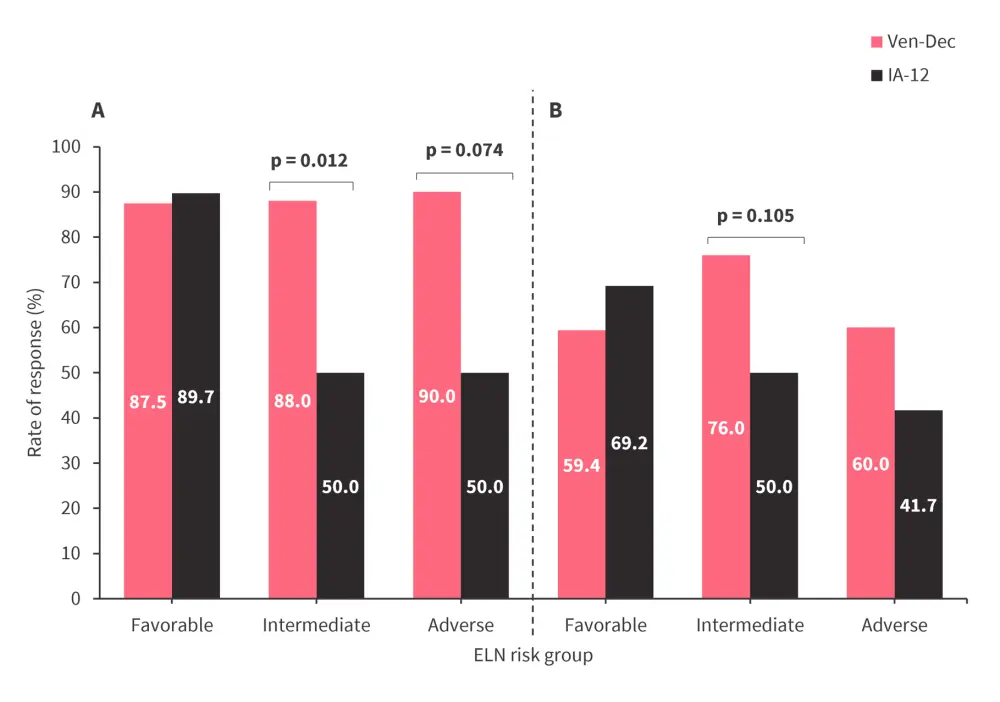
CRc, composite complete remission; ELN, European LeukemiaNet; IA-12, idarubicin and cytarabine; MRD, measurable residual disease; Ven-Dec, venetoclax plus decitabine.
*Adapted from Lu.1
- Subgroup analysis of CRc rate after the second induction cycle showed a significant benefit of Ven-Dec vs IA-12 in patients aged ≥40 years (95% vs 69.4%; p = 0.005) and patients with a blast burden of 30–50% (100% vs 68.8%; p = 0.006).
Safety
- Ven-Dec was well tolerated vs IA-12 (Table 1).
Table 1. Safety analysis by treatment received*
|
IA-12, idarubicin and cytarabine; PLT, platelet; RBC, red blood cell; Ven-Dec, venetoclax plus decitabine. |
|||
|
Adverse events, % (unless otherwise specified) |
Ven-Dec |
IA-12 |
p-value |
|---|---|---|---|
|
30-day mortality |
0 |
1.5 |
— |
|
100-day mortality |
1.5 |
4.5 |
0.362 |
|
Fever |
|
|
|
|
Any-grade |
53.7 |
82.1 |
<0.01 |
|
Grade ≥3 |
9.0 |
26.9 |
0.012 |
|
Febrile neutropenia |
|
|
|
|
Grade ≥3 |
37.3 |
74.6 |
<0.01 |
|
Infection |
|
|
|
|
Grade ≥3 |
26.8 |
67.2 |
<0.01 |
|
Sepsis |
4.5 |
29.9 |
<0.01 |
|
Pneumonia |
19.4 |
32.8 |
0.115 |
|
Mean duration of thrombocytopenia, day (range) |
12.4 (0–42) |
20.2 (4–47) |
<0.01 |
|
Mean duration of neutropenia, day (range) |
22.9 (0–45) |
20.8 (1–47) |
0.19 |
|
Mean RBC transfusion, units (range) |
14 (0–44) |
17.6 (0–48) |
0.05 |
|
Mean PLT transfusion, units (range) |
46 (0–130) |
78 (30–270) |
<0.01 |
Survival
- At a median follow-up of 7.5 months, median overall survival was not reached in either treatment arm, and median event-free survival was not reached in the Ven-Dec arm vs 10.8 months in the IA-12 arm.
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


