All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
MRD response in patients with newly diagnosed AML treated with venetoclax and azacitidine in the VIALE-A trial
The phase III VIALE-A trial (NCT02993523) is a randomized, double-blind study investigating the use of venetoclax + azacitidine (Ven+Aza) for the treatment of patients with newly diagnosed acute myeloid leukemia (AML). This study has previously been reported on the AML Hub and demonstrated that patients treated with Ven+Aza had higher rates of composite complete remission (CRc; complete remission [CR] + CR with incomplete hematologic recovery) and measurable residual disease response (MRD <10−3) compared with those treated with azacitidine alone (23.4% vs 7.6%; p < 0.001).
As there is limited evidence of the clinical significance of MRD monitoring in patients receiving low-intensity chemotherapy, Keith Pratz and colleagues investigated the prognostic impact of MRD <10−3 on the outcome of patients from the VIALE-A study who were treated with Ven+Aza. The results of this study were presented during the European Hematology Association (EHA)2021 Virtual Congress and are summarized below.
Study design and patient characteristics1
- Enrolled patients were ≥18 years of age and unfit for intensive chemotherapy.
- Dosage: Venetoclax 400 mg orally on Days 1–28; azacitidine 75 mg/m2 on Days 1–7/28-day cycle.
- MRD was assessed from bone marrow aspirates taken at baseline, end of Cycle 1, and every 3 cycles thereafter, by multiparametric flow cytometry using the integrated leukemia-associated immunophenotypes and different than normal method.
- Outcomes evaluated: Duration of remission (DoR) for CRc, overall survival (OS), and event-free survival (EFS).
- Of the 286 patients treated with Ven+Aza, 164 patients had a CRc and had samples evaluable for MRD analysis.
- Of these patients, 67 (41%) achieved MRD <10−3, while 97 (59%) had an MRD ≥10−3.
- Baseline characteristics of the patients included in this analysis can be seen in Table 1.
- MRD responses were seen in all subsets of patients.
- Of note, MRD response was seen in 33% of patients with poor risk cytogenetics, 30% of patients with TP53 mutations, and 88% of patients with NMP1 mutations.
Table 1. Baseline patient characteristics*
|
*Data from Pratz et al.1 |
||
|
Characteristic, n |
CRc + MRD <10−3 |
CRc + MRD ≥10−3 |
|---|---|---|
|
Age group, years |
|
|
|
18 to <65 |
2 |
1 |
|
65 to <75 |
27 |
26 |
|
≥75 |
38 |
70 |
|
Gender, male/female |
34/33 |
64/36 |
|
ECOG performance score |
|
|
|
0–1 |
38 |
63 |
|
2–3 |
29 |
34 |
|
Cytogenetic risk |
|
|
|
Intermediate |
51 |
65 |
|
Poor |
16 |
32 |
|
Type of AML |
|
|
|
De novo AML |
49 |
71 |
|
Secondary AML |
18 |
26 |
|
Molecular mutations |
|
|
|
FLT3 ITD/TKD |
10 |
10 |
|
IDH1/2 |
21 |
22 |
|
TP53 |
6 |
14 |
|
NPM1 |
15 |
2 |
Results1
- Median follow-up was 22.1 months for patients who achieved MRD <10−3 and 20.8 months for those with CRc + MRD ≥10−3.
- Patients with an MRD response were treated for a median of 16 cycles, while those with MRD ≥10−3 had a median of 9 cycles.
- Median DoR, EFS, and OS were not reached in patients with an MRD response (Table 2).
- 18-month estimates for DoR, EFS, and OS were better for those who achieved an MRD response compared with those who did not.
Table 2. Responses*
|
*Data from Pratz et al.1 CI, confidence interval; CRc, composite complete response; DoR, duration of response; EFS, event free survival; MRD, minimal residual disease; NR, not reached; OS, overall survival. |
||||
|
Response |
18-month estimate, % (95% CI) |
Median response, months (95% CI) |
||
|---|---|---|---|---|
|
CRc + MRD <10−3 |
CRc + MRD ≥10−3 |
CRc + MRD <10−3 |
CRc + MRD ≥10−3 |
|
|
DoR |
69.6 (55.9–79.8) |
33.5 (22.9–44.5) |
NR (19.3–NR) |
9.7 (8.0–15.8) |
|
EFS |
73.7 (61.1–82.8) |
33.9 (24.4–43.6) |
NR (19.7–NR) |
10.6 (9.0–13.9) |
|
OS |
84.6 (73.3–91.4) |
50.1 (39.6–59.8) |
NR (24.4–NR) |
18.7 (12.9–NR) |
- Multivariate Cox regression analysis of OS revealed two covariates associated with OS; MRD response (hazard ratio, 0.285; 95% confidence interval, 0.159–0.510; p < 0.001) and cytogenetic risk (hazard ratio, 2.062; 95% confidence interval, 1.260–3.374; p = 0.004)
- The timing of MRD response generally occurred later than achievement of clinical response.
- 25% of patients achieved an MRD response by the end of Cycle 1.
- 21% of patients achieved an MRD response by the end of Cycle 7.
- Patients who achieved MRD response had a higher rate of ≥Grade 3 febrile neutropenia, neutropenia, and thrombocytopenia compared with those who did not achieve an MRD response (Figure 1).
- Serious adverse events were similar across both groups.
Figure 1. Adverse events*
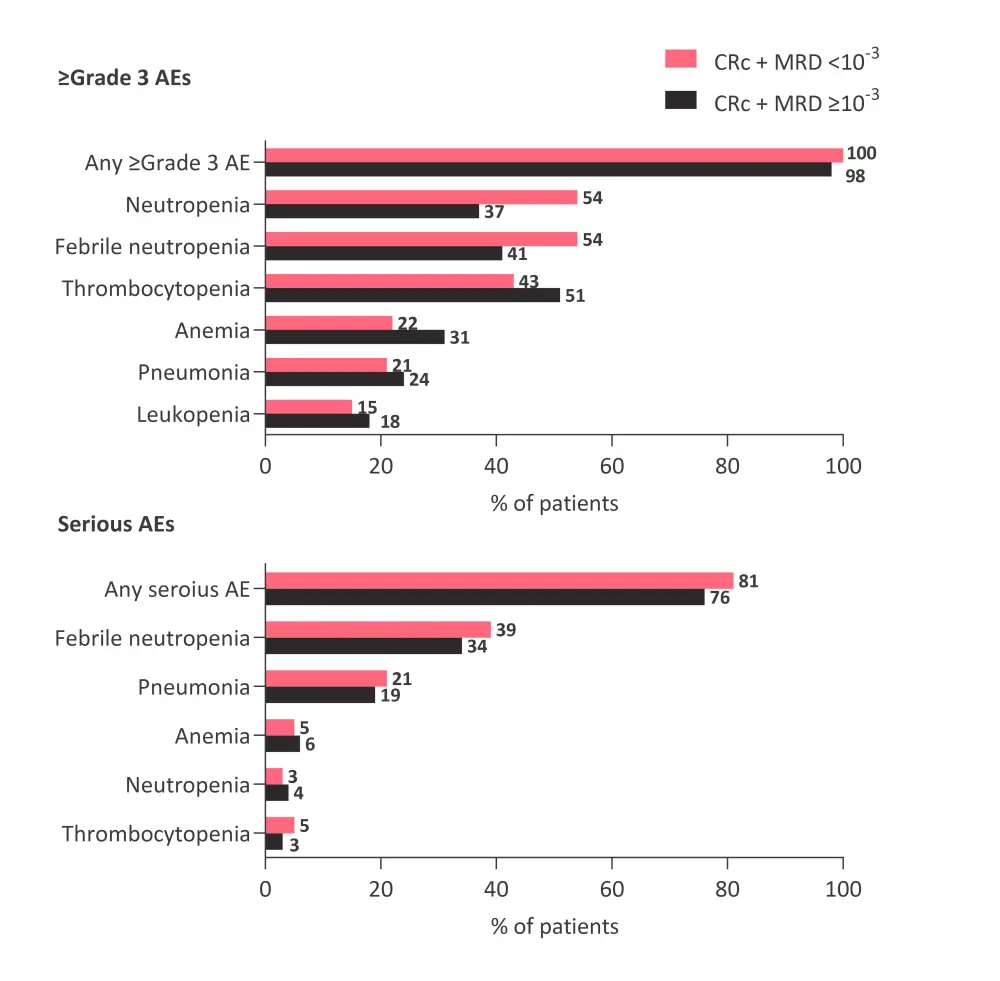
*Adapted from Pratz et al.1
AE, adverse event; CRc, composite complete response; MRD, minimal residual disease.
Conclusion
Patients with newly diagnosed AML treated with Ven+Aza who achieved CRc and an MRD response <10−3 had a longer DoR, EFS, and OS compared with those who did not achieve an MRD response (≥10−3). Higher rates of neutropenia were observed in patients that achieved an MRD response compared with those who did not. MRD response was also a significant predictor of OS, however, further studies are required to investigate the role of MRD response in clinical management.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

