All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
VIALE-A trial update | Venetoclax and azacitidine combination for the treatment of older patients with newly diagnosed AML
Featured:
VIALE-A (NCT02993523) is a randomized, double-blind, multicenter, placebo-controlled phase III study that compares venetoclax and azacitidine (Ven + Aza) versus placebo + Aza in patients with acute myeloid leukemia (AML) who are not eligible for intensive induction therapy. AML develops mostly in older populations (with a median age between 68–72 years) where treatment options are limited, and survival is poor for patients who are aged ≥ 75 years or have severe comorbidities. These patients are also poor candidates for intensive induction chemotherapy. Low-dose azacitidine, a hypomethylating agent, is associated with low response rates, a longer time to response, and a median overall survival of less than a year. Venetoclax is a highly specific, oral BCL-2 inhibitor for managing overexpression of anti-apoptotic BCL-2 in AML and the AML stem cell population. The combination of venetoclax and hypomethylating agents has been associated with clinical activity in older, treatment-naïve patients with AML. Courtney DiNardo, The University of Texas MD Anderson Cancer Center, Houston, US, presented the results of the VIALE-A study during the 25th European Hematology Association (EHA) Annual Congress, held virtually on June 14, 2020.1
Study Design1
- Primary endpoints: Overall survival and composite complete remission rate (complete remission [CR] plus CR with incomplete marrow remission [CRi])
- Secondary endpoints: Event-free survival, CR plus CR with partial hematologic recovery (CRh), transfusion independence, and patient-reported outcomes
The detailed study design can be found here.
Patient characteristics1
- Median age was 76 years in both treatment groups, and the proportion of patients ≥ 75 years was similar (61% in the Ven + Aza group; 60% in the placebo + Aza group)
- Patient characteristics were well balanced between treatment groups in terms of AML type (de novo or secondary), Eastern Cooperative Oncology Group (ECOG) performance status (0–1 and 2–3), bone marrow blast count, and poor/intermediate cytogenetic risk
- The proportion of patients with AML with or without myelodysplasia-related changes was similar between treatment groups
- The profile of somatic mutations is shown below in Figure 1
Figure 1. The distribution of somatic mutations among groups
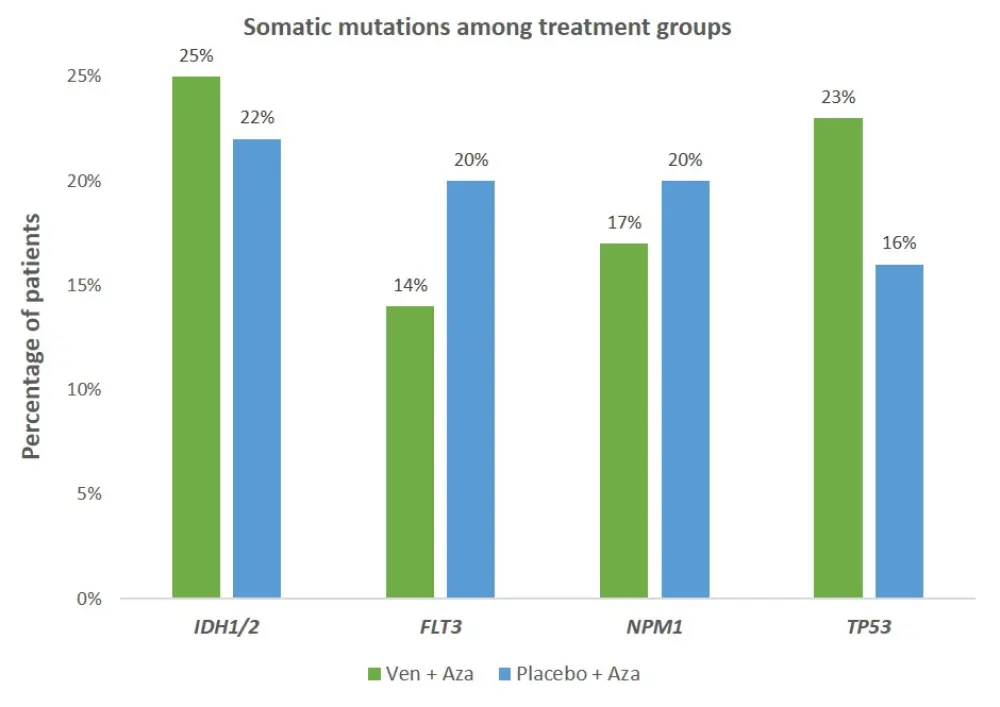
As of cutoff date, Jan 4, 2020:
- Median follow-up duration for the Ven + Aza group and the placebo + Aza group was 20.7 months (range, 0.0–30.7) and 20.2 months (0.2–28.8), respectively
- The number of patients still receiving treatment was 77 (27%) for the Ven + Aza group and 18 (12%) for the placebo + Aza group
- A total of 336 patients discontinued treatment (209 in the Ven + Aza group and 127 in the placebo + Aza group), mostly due to disease progression or relapse (120 vs 62), withdrawn consent (26 vs 22), physician’s decision (17 vs 9), and death (39 vs 23)
- The main reason for study discontinuation was death (161 in the Ven + Aza group vs 109 in the placebo + Aza group)
Results1
The combination of venetoclax and azacitidine showed a 34% reduction in risk of death (HR, 0.66; 95% CI, 0.52–0.85; p < 0.001) at 20.5 months follow-up compared to azacitidine alone. Median overall survival in the Ven + Aza and placebo + Aza groups were 14.7 and 9.6 months, respectively. Subgroup analyses showed consistent treatment effects and better response rates of CR + CRi in favor of the combination. The combination therapy was associated with substantially higher response rates in all somatic mutations analyzed (see Figure 2). The proportion of patients with transfusion independence was higher in Ven + Aza group compared to placebo + Aza (p < 0.001). The summary of study results and safety analysis are presented in Table 1 and Table 2.
Table 1. Study results
|
AML, acute myeloid leukemia; Aza, azacitidine; CR, complete remission; CRh, CR with partial hematologic recovery; CRi, CR with incomplete marrow remission; ECOG, Eastern Cooperative Oncology Group; RBC, red blood cell; Ven, venetoclax *HR, 0.63; 95% CI, 0.50–0.80; p < 0.001) |
||
|
Outcome |
Ven + Aza n = 286 |
Placebo + Aza n = 145 |
|---|---|---|
|
Median number of cycles, n (range) |
7.0 (1.0–30.0) |
4.5 (1.0–26.0) |
|
Composite response rate (CR + CRi), %
|
66.4
|
28.3
|
|
Median time to CR/CRi, months (range) |
1.3 (0.6–9.9) |
2.8 (0.8–13.2) |
|
Median duration of CR/CRi, months |
17.5 |
13.4 |
|
Response rate by CR + CRh, % |
64.7 |
22.8 |
|
Median time to CR/CRh, months (range) |
1.0 (0.6–14.3) |
2.6 (0.8–13.2) |
|
Median duration of CR/CRh, months |
17.8 |
13.9 |
|
Response rates (CR + CRi) by subgroup, % Cytogenetic risk Intermediate Poor AML subtype De novo Secondary Age < 75 years ≥ 75 years ECOG score < 2 ≥ 2 Bone marrow blasts 20 to < 30% ≥ 30 to < 50% ≥ 50% |
74 53
66 67
63 69
69 64
77 57 64 |
32 23
30 23
41 20
25 33
39 27 23 |
|
Transfusion independence RBC Platelet RBC and platelet |
60 69 58 |
35 50 34 |
|
Median event-free survival, months (range)* |
9.8 (8.4–11.8) |
7.0 (5.6–9.5) |
Figure 2. Response rates (CR + CRi) by somatic mutations
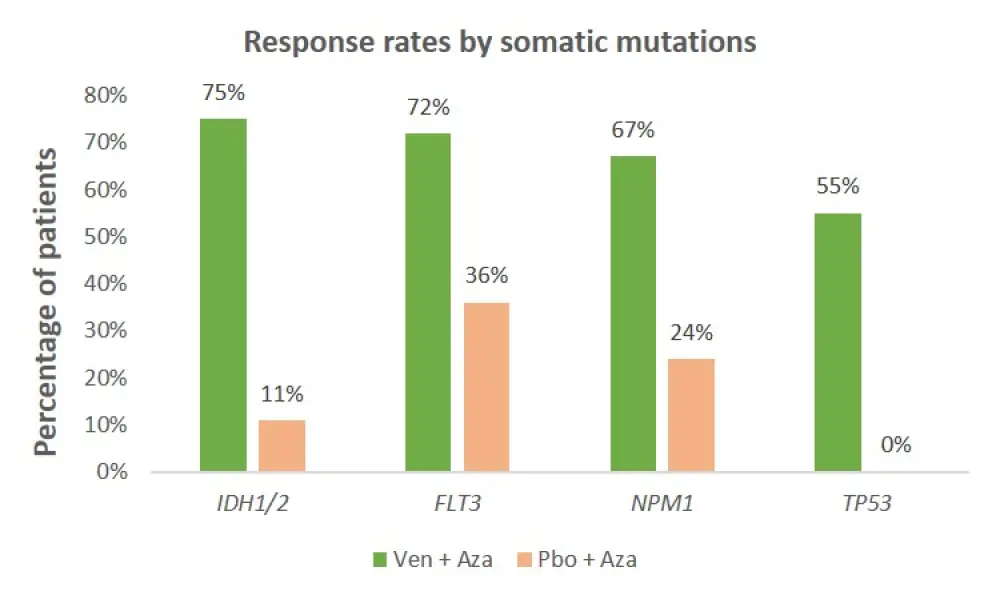
Table 2. The results of safety analysis (as of Jan 4, 2020)
|
AE, adverse event; Aza, azacitidine; Ven, venetoclax *≥ 10% occurrence †≥ 5% occurrence |
||
|
|
Ven + Aza n = 283 |
Placebo + Aza n = 144 |
|---|---|---|
|
All Grade 3/4 AEs*, n (%) |
279 (99) |
139 (97) |
|
Grade 3/4 hematologic AEs Thrombocytopenia Neutropenia Febrile neutropenia Anemia Leukopenia |
233 (82) 126 (45) 119 (42) 118 (42) 74 (26) 58 (21) |
98 (68) 55 (38) 41 (29) 27 (19) 29 (20) 17 (12) |
|
Grade 3/4 non-hematologic AEs* Diarrhea Hypokalemia |
46 (17) 13 (5) 30 (11) |
44 (31) 4 (3) 15 (10) |
|
Serious AEs† Febrile neutropenia Pneumonia |
235 (83) 84 (30) 47 (17) |
105 (73) 15 (10) 32 (22) |
|
Deaths† ≤ 30 days after first dose ≤ 60 days after first dose |
21 (7) 43 (15) |
9 (6) 24 (17) |
Neutropenia, febrile neutropenia, and thrombocytopenia were the most common adverse events leading to dose interruptions and occurred in more patients in the Ven + Aza group compared to the placebo + Aza group (204 [72%] vs 82 [57%]).
Conclusion
The results showed that Ven + Aza combination therapy was associated with higher response rates in difficult-to-treat patient groups, such as older patients (≥ 75 years) or those with poor cytogenetic risk. Patients in the combination group reached a faster and more durable response compared to those in the azacitidine alone group. The safety profile was manageable and similar to previous clinical experience. The combination of azacitidine and venetoclax demonstrated both statistically and clinically meaningful improvement in overall survival, response rates, and transfusion independence. Response rates were particularly striking for combination treatment in the subgroup analysis of patients with deleterious somatic mutations, e.g., IDH1, potentially providing a new treatment modality for a personalized medicine approach.
Expert Opinion
 Courtney DiNardo
Courtney DiNardoReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

