All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Efficacy and safety of eprenetapopt (APR-246) combined with azacitidine in patients with TP53-mutant MDS and oligoblastic AML
Around 20% of patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) and 30–40% of patients with therapy-related myeloid diseases have TP53 mutations.1 These patients have a worse prognosis and very poor outcomes, including inferior overall survival (OS), increased risk of AML transformation, a variant allele frequency (VAF) of >40%, and biallelic mutations. Targeted therapies, such as venetoclax in combination with hypomethylating agents (HMA), show a complete response (CR) rate of about 20%, but survival rates remain low in this molecularly distinct cohort of patients. Eprenetapopt (APR-246), a p53 reactivator, has recently demonstrated promising efficacy and safety in two phase II trials (NCT03072043 and NCT03588078) when combined with azacitidine.1 The MDS Hub has previously reported on these early phase trials.
During the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, David Sallman1 presented the updated findings from intention-to-treat (ITT) analyses from the combined cohorts of these two phase II trials. Here, we summarize the key findings.
Study design
The study comprised ITT analyses of the combined phase II cohorts from the US MDS Clinical Research Consortium and the Groupe Francophone des Myélodysplasies (GFM) trials. Enrolled patients were TP53-mutant with either HMA-naïve MDS, oligoblastic AML (≤30% blasts), or MDS–myeloproliferative neoplasm (MDS–MPN). The US trial excluded patients with ≥30% blasts. The treatment schedule is shown in Figure 1.
The primary endpoint was CR rate according to International Working Group (IWG) 2006 criteria. Secondary endpoints included the following:
- Overall response rate (ORR)
- Duration of response (DoR)
- OS
- Serial high-depth next-generation sequencing (NGS) with a VAF 0.1% cut-off for evaluation of measurable residual disease (MRD)
Figure 1. Treatment schedule*
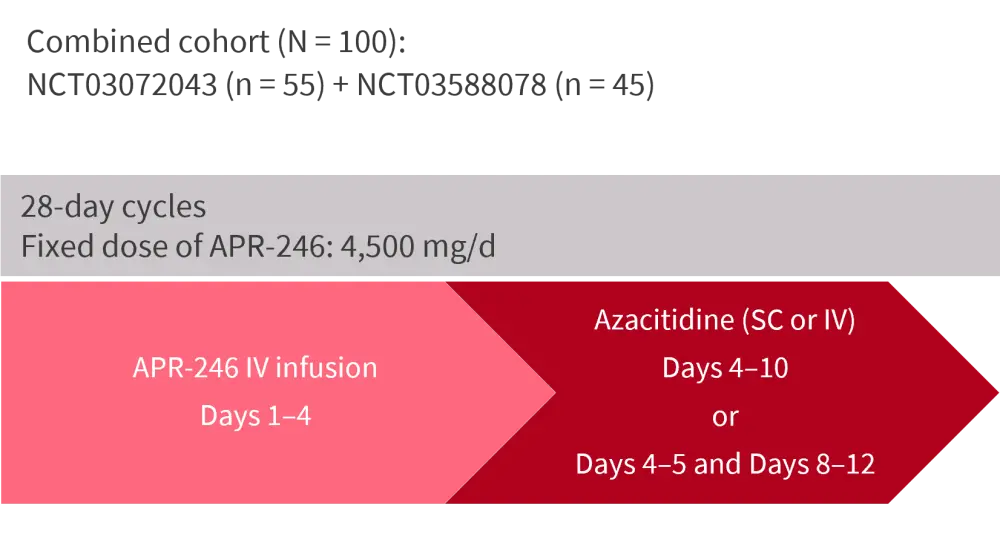
APR-246, eprenetapopt; d, day; IV, intravenous; SC, subcutaneous.
*Adapted from Sallman, et al.1
Results
Baseline characteristics
A total of 100 patients were included: 53% were female. Baseline characteristics are shown in Table 1.
- The cutoff date was July 15, 2021.
- The median time on treatment was six cycles (range, 1–25) with five patients ongoing treatment at data cutoff.
- 23 patients had proceeded to allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Table 1. Baseline characteristics*
|
AML, acute myeloid leukemia; CMML; chronic myelomonocytic leukemia; IPSS-R, revised International Prognostic Scoring System; MDS, myelodysplastic syndromes; MPN, myeloproliferative neoplasm; VAF, variant allele frequency. *Adapted from Sallman, et al.1 |
||||
|
Characteristic, % unless otherwise stated |
Total |
MDS |
AML |
MDS–MPN/CMML |
|---|---|---|---|---|
|
Median age, years (range) |
68 (34–87) |
68 (34–87) |
68 (47–85) |
57 (41–79) |
|
MDS |
74 |
|
|
|
|
IPSS-R: Intermediate |
- |
7 |
- |
- |
|
IPSS-R: High |
- |
15 |
- |
- |
|
IPSS-R: Very high |
- |
78 |
- |
- |
|
AML |
22 |
- |
- |
- |
|
MDS–MPN/CMML |
4 |
- |
- |
- |
|
Therapy related |
22 |
24 |
18 |
0 |
|
Isolated TP53 mutation |
63 |
64 |
59 |
75 |
|
Median TP53 VAF, range |
22 (1–83) |
25 (1–83) |
18 (1–66) |
46 (22–47) |
|
Biallelic and/or complex karyotype |
88 |
88 |
87 |
100 |
Efficacy
- At a median follow-up of 27.8 months, 69% of patients attained ORR (Table 2).
- CR/PR was 49%, 36%, and 0% with an ORR of 70%, 64%, and 75% in patients with MDS, AML, and MDS–MPN, respectively.
- Isolated TP53 mutations were predictive of a higher CR rate compared with other non-isolated mutations (52% vs 30%; p = 0.04).
- A higher CR rate was also significantly associated with the presence of biallelic TP53 or complex karyotype compared to their absence (49% vs 8%; p = 0.01)
- 40 patients achieved NGS negativity, including MRD negativity in six patients (Table 2).
- 78% NGS-negative patients achieved a CR/PR.
- All six MRD-negative patients received at least five cycles of treatment.
- The median number of cycles in patients who achieved TP53 negativity and TP53 positivity was ten and four, respectively (p < 0.0001).
- Median OS was:
- Significantly improved at 11.8 months (95% confidence interval [CI], 9.4–14.3) in all patients and was 16.1 months (95% CI, 14–18.1) in patients undergoing allo-HSCT.
- Improved in patients achieving CR/PR or TP53 negativity (15.8 vs 10.1 months; p = 0.0019) compared to those not achieving CR/PR or TP53 negativity.
- Improved in patients achieving CR/PR/TP53 negativity prior to allo-HSCT compared to those who did not achieve this response prior to allo-HSCT (not reached vs 9.1 months, respectively; p = 0.02)
- Patients achieving MRD negativity had a 2-year OS of 50% vs 23% in MRD-positive patients (p = 0.21).
Table 2. ITT analysis: best responses*
|
BAT, best response to therapy; CI, confidence interval; CR, complete response; DoR, duration of response; HI, hematological improvement; mCR, marrow complete response; MLFS, morphological leukemia-free state; MRD, minimal residual disease; NE, not evaluable; NGS, next-generation sequencing; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease; VAF, variant allele frequency. *Adapted from Sallman, et al.1 |
|
|
Response, % unless otherwise stated |
Total (N = 100) |
|---|---|
|
ORR |
69 |
|
BAT |
|
|
CR |
43 |
|
PR |
1 |
|
mCR + HI |
10 |
|
mCR/MLFS |
6 |
|
HI |
9 |
|
SD |
6 |
|
NE |
18 |
|
PD |
7 |
|
CR/PR |
44 |
|
Median time to CR/PR, months (range) |
3.1 (2.5–6.7) |
|
Median DoR, months (95% CI) |
10.6 (8.8–12.3) |
|
TP53 |
|
|
NGS negative (5% VAF cutoff) |
40 |
|
MRD negativity (0.1% cutoff) |
6 |
Safety
- Treatment-emergent adverse events (TEAEs) occurred in ≥20% of patients, with particularly increased nausea and vomiting in 58% of patients.
- 26% and 23% of patients also experienced ataxia and dizziness, respectively.
- The most common serious AEs were febrile neutropenia (37%) and neurological events (40%).
- 30- and 60-day mortality were low at 1% and 7%, respectively.
- Dose reductions of eprenetapopt and azacitidine occurred in 16% and 1% of patients, respectively, with only one treatment discontinuation due to a TEAE.
Conclusion
This ITT analyses from the combined cohort demonstrated the feasibility and efficacy of using eprenetapopt with azacitidine for the treatment of patients with TP53-mutant MDS or AML. Eprenetapopt plus azacitidine showed an encouraging safety profile and promising response rates. The study identified molecular markers associated with CR and validated NGS clearing as a critical biomarker for allo-HSCT outcomes in patients with TP53 mutations. Results from the study support the phase III trial (NCT03745716) comparing eprenetapopt plus azacitidine versus azacitidine alone for the treatment of patients with TP53-mutant MDS. However, this trial did not meet its primary endpoint of significantly improving CR rates.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

