All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
An overview of developments in immune-based therapies in the treatment of AML and MDS: Updates from #ASCO23
Acute myeloid leukemia (AML) is a heterogenous disease associated with a broad continuum of molecular changes. In the past decade, there have been advances in novel treatment strategies including immunotherapies that harness the power of T-cells against AML. These include bispecific and dual antigen receptor-targeting antibodies (targeting CD33, CD123), chimeric antigen receptor (CAR) T-cell therapies, and T-cell immune checkpoint inhibitors (targeting PD-1, PD-L1, CTLA-4).1
During the American Society of Clinical Oncology (ASCO) Meeting 2023, the progress of several ongoing trials evaluating the efficacy and safety of T-cell-based immunotherapies were presented. We are pleased to present a summary of three poster presentations on: the efficacy and safety of vibecotamab by Nicholas Short2; the antileukemic activity and safety of pivekimab sunirine (PVEK) by Naval Daver3; and a first-in-human trial of tocilizumab by Pierre Peterlin.4
Developments in immunotherapies
Most patients with AML or myelodysplastic neoplasms (MDS) express CD123.2 On normal hematopoietic progenitors, CD123 is expressed at incredibly low levels, making it a potentially effective therapeutic target.
Vibecotamab2
Vibecotamab is a CD3-CD123 bispecific T-cell-engaging antibody (Figure 1) demonstrating clinical activity in patients with relapsed/refractory (R/R) AML. In a phase I study, vibecotamab (dose ≥0.75 µg/kg) demonstrated an overall response rate (ORR) of 26% in patients with ≤25% blasts.
Nicholas Short presented the progress of an open-label, phase II trial of vibecotamab (NCT05285813) in patients with positive measurable residual disease (MRD+) AML, MDS, or chronic myelomonocytic leukemia (CMML) after failure with hypomethylating agents. Patients with either an MRD level of ≥0.1% (by multiparameter flow cytometry), or intermediate/higher-risk MDS, or CMML-1 or CMML-2 are eligible. Enrolled patients are given vibecotamab intravenously (IV) on Days 1 (0.43 µg/kg), 3 (0.75 µg/kg), 5 (1.1 µg/kg), and 8 (1.7 µg/kg) of Cycle 1, followed by a weekly dose of vibecotamab 1.7 µg/kg. The primary endpoint in the MRD+ cohort is the determination of the MRD negativity rate (target 50%), and in the MDS/CMML cohort it is ORR (target 30%) after 4 cycles of vibecotamab.
The trial started in May 2022 and is actively recruiting patients at the MD Anderson Cancer Center. To date, 17 patients have been enrolled, 9 with MRD+ AML and 8 with MDS or CMML. Active recruitment of patients is planned until the end of 2024, with an aim of 20 patients per cohort.
Figure 1. Structure of vibecotamab*
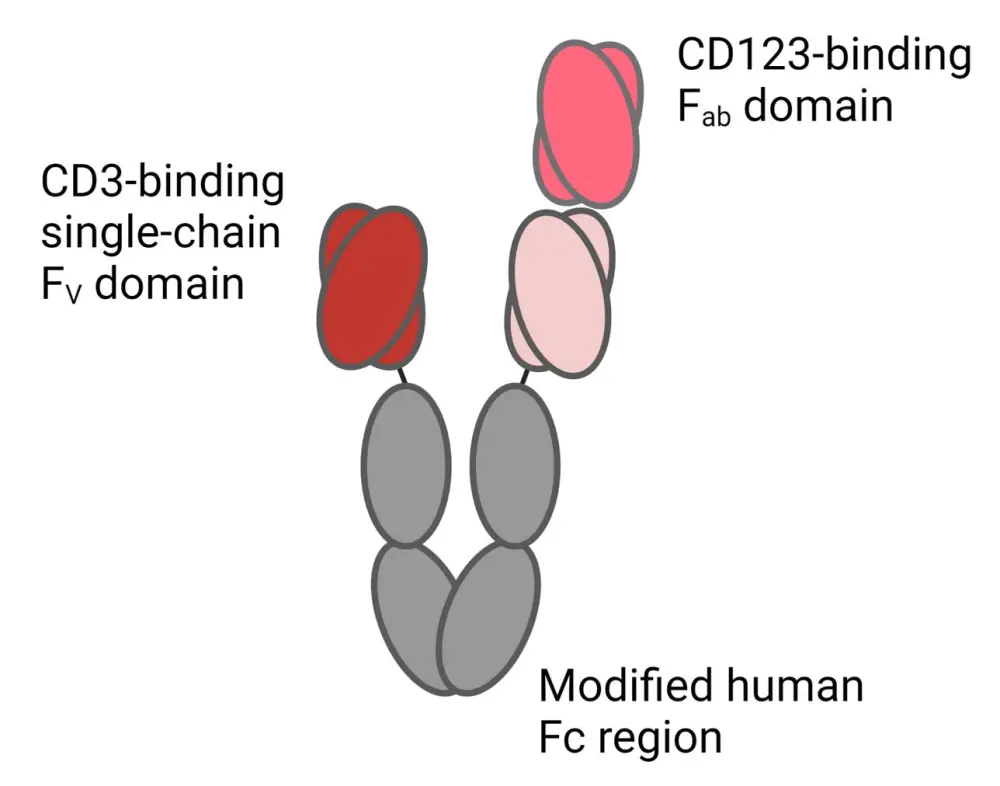
*Adapted from Short.2 Created with BioRender.com.
Pivekimab sunirine3
Long-term survival is inadequate in patients with frontline unfit AML, despite improved outcomes with azacitidine (AZA) and venetoclax (VEN) treatment. Pivekimab sunirine (PVEK) is an antibody–drug conjugate (ADC) that contains an indolinobenzodiazepine pseudodimer payload and a cleavable linker and is a high-affinity CD123 antibody (Figure 2A). According to pre-clinical studies, PVEK in combination with AZA and VEN has demonstrated therapeutic synergy. Clinical evidence also supports continued research into PVEK, AZA, and VEN as a combination therapy. Magrolimab is a monoclonal antibody blocking CD47 (Figure 2B). Magrolimab has shown promise as a monotherapy therapeutic for R/R AML in a phase I study.
Figure 2. A The Structure and mechanism of action of pivekimab sunirine and B the structure and mechanism of action of magrolimab*
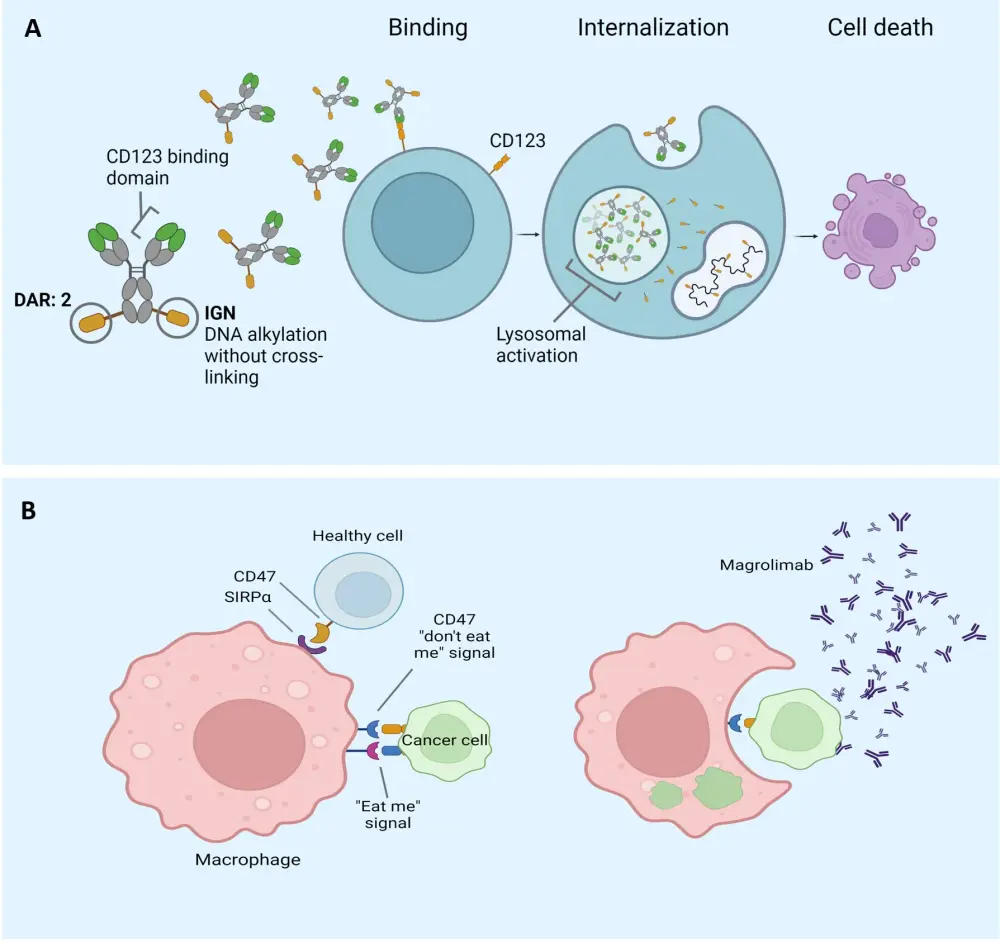
*Adapted from Daver.3 Created with BioRender.com.
The trial is comprised of two cohorts:
- Cohort C (PVEK + AZA + VEN regimen) in patients with newly diagnosed CD123+ AML who are unfit for intensive induction therapy.
- In Cohort C, patients will receive the established recommended phase II dose (RP2D) of PVEK 0.045 mg/kg IV (<30-minute outpatient infusion) plus AZA (75 mg/m2 SC or IV) on Days 1–7, and VEN (up to 400 mg PO) daily for 14 days (Cohort 1) or 28 days (Cohort 2).
- Cohort E (PVEK + magrolimab regimen) in patients with R/R CD123+ AML and hemoglobin ≥9 g/dL prior to initial dose of magrolimab.
- In Cohort E, patients will receive PVEK (IV) on a dose-escalation schedule on Day 1 of the 28-day cycles combined with 30 mg/kg of magrolimab every 2 weeks, after the standard ramp-up schedule.
The primary and key secondary endpoints are listed below:
- Cohort C primary endpoints: antileukemic activity assessed by composite CR rate, ORR, and duration of remission, and MRD levels as assessed by central flow cytometry.
- Cohort E primary endpoints: dose-limiting toxicity (DLT) in the safety phase; and antileukemic activity, as assessed by composite CR rate, ORR, and duration of CR, in the expansion phase.
- Cohort C and E key secondary endpoints: safety and tolerability, pharmacokinetics, and immunogenicity.
Cohort C is currently enrolling patients across 29 sites in France, Germany, Italy, Spain, UK, and USA. Cohort E will open for enrollment mid-2023 at the US sites.
Tocilizumab4
Interleukin-6 promotes resistance to chemotherapy and is known to be related to poor prognosis in patients with AML. IL-6 blockade may therefore represent a promising new therapeutic strategy for the management of AML.
Pierre Peterlin presented the early findings from the phase I TOCILAM single-center trial (NCT04547062). This is a first-in-human trial of tocilizumab in combination with standard induction chemotherapy in patients with newly diagnosed AML with a favorable risk, or R/R AML. Three doses of tocilizumab were evaluated: 4, 6, and 8 mg/kg administered on Day 8 of a standard AML induction chemotherapy regimen of idarubicine 8 mg/m2 on Days 1–5 plus cytarabine 100 mg/m2 on Days 1–7. The primary aim of the trial was to determine the maximum tolerated dose of tocilizumab to inform phase II and III studies.
Key findings
A total of 12 patients were included between December 2020 and 2022 with a median age of 66 years (range, 65–70.5 years) and a median follow-up of 675 days.
- No patients reported DLT from tocilizumab.
- The median levels of IL-6 increased after tocilizumab from 23.2 pg/mL to 202.9 pg/mL and 481.5 pg/mL on Day 8 and 15, respectively.
- The median levels of tocilizumab were also higher at 8 and 15 days after injection: 34.8 µg/ml at the 6 mg/kg dose and 38.5 µg/ml at the 8 mg/kg dose compared with 10.9 µg/ml at 4 mg/kg dose.
- The RP2D is 8 mg/kg of tocilizumab.
- The overall remission rate for evaluable patients was 90%.
- The most common Grade ≥3 adverse events were bacteremia occurring in 67% and pneumonia in 25% of patients.
- Nine serious adverse events were reported; none were related to tocilizumab.
- Two patients died, one at Day 21 from an infection and the other at Day 15 from a cerebral hemorrhage.
Conclusion
The range of ongoing trials evaluating various immune-based therapeutics for the treatment of AML are promising. The TOCILAM study demonstrated that the combination of tocilizumab and standard intensive induction therapy for AML appears to be safe, including higher ORR and the expected biological activity. Further findings from these trials will inform the efficacy and safety data of immune-based therapies in the treatment of AML.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

