All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
The prognostic impact of FLT3-ITD mutations in CBF-AML
Do you know... In terms of overall survival (OS), allo-HSCT is beneficial compared with chemotherapy for which of the following patient groups?
Positive outcomes reported in patients with core-binding factor (CBF) fusions such as t(8;21)(q22;q22) or inv(16) (p13.1q22)/t(16;16) (p13.1;q22) have led to the creation of a distinct molecular subclass of acute myeloid leukemia (AML) by the World Health Organization (WHO). Moreover, CBF-AML is classified as ‘favorable risk’ by both the European LeukemiaNet guidelines and the National Comprehensive Cancer Network. Research indicates that there may not be any survival benefit of allogeneic hematopoietic stem cell transplantation (allo-HSCT) over chemotherapy in patients with CBF-AML in first complete remission (CR1); however, the impact of FLT3-internal tandem duplication (ITD) mutations, which occur in up to 10% of patients, has not been fully investigated.1
In a recent multicenter study published by Kayser et al.1 in Haematologica, patients with FLT3-ITD CBF-AML were characterized and treatment outcomes were compared, particularly with respect to allo-HSCT. We summarize key findings below.
Methods
This large, multicenter study investigated case reports from patients with CBF-AML and FLT3-ITD mutations from 1996 to 2019 across eight study groups/institutions in the US and Europe (Figure 1).
Figure 1. Study design*
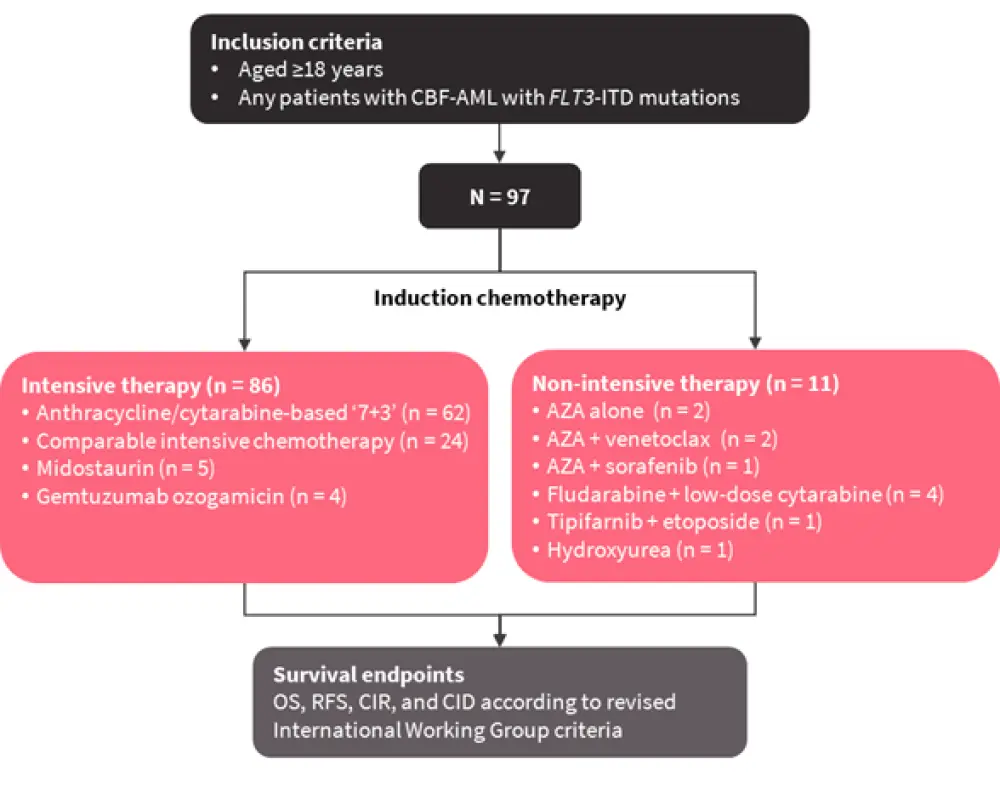
AZA, azacitidine; CBF-AML, core-binding factor acute myeloid leukemia; CID, cumulative incidence of death in complete remission; CIR, cumulative incidence of relapse; OS, overall survival; RFS, relapse-free survival.
*Adapted from Kayser et al.1
Results
Patient characteristics are summarized in Table 1.
Table 1. Patient characteristics*
|
AML, acute myeloid leukemia; BM, bone marrow; CBF, core-binding factor; Hb, hemoglobin; ITD, internal tandem duplication; TKD, tyrosine kinase domain; WBC, white blood cell. |
|
|
Characteristic |
N = 97 |
|---|---|
|
Median age, years (range) |
53 (19–81) |
|
Female, % |
46 |
|
Median WBC, × 109/L (range) |
20.5 (1.8–298) |
|
Median Hb, g/dL (range) |
8.6 (4.6–14.3) |
|
Median platelet count, × 109/L |
33 (7–372) |
|
Median BM blasts, % (range) |
60 (0–98) |
|
Cytogenetics, % |
|
|
CBF as sole abnormality |
42 |
|
CBF + other abnormality |
58 |
|
Trisomy 22 |
12 |
|
Trisomy 8 |
7 |
|
Disease type, % |
|
|
De novo AML |
90 |
|
Secondary AML |
2 |
|
Therapy-related AML |
8 |
|
Median FLT3-ITD allelic ratio (range) |
0.35 (0.003–50) |
|
FLT3-TKD, % |
21 |
Cytogenetic and molecular analysis
- Out of 97 patients, the prevalence of balanced translocation t(8;21)(q22;q22) was 52%
- This was the sole abnormality in 30% patients
- Other common cytogenetic abnormalities were loss of a sex chromosome (n = 26), ≥3 other abnormalities (n = 10), and del(9q) (n = 6)
- A total of 48% of patients harbored inv(16)(p13q22) (n = 46) or t(16;16)(p13;q22) (n = 1)
- These were the sole abnormality in 53% of patients
- The most frequent concurrent abnormalities were trisomy 22 (n = 11), ≥3 abnormalities (n = 7), trisomy 8 (n = 8), and monosomy 7 or del(7q) (n = 4)
Allo-HSCT
- Overall, allo-HSCT was performed in 24 patients (25% of the cohort), including:
- 12 patients in CR1: Four with inv(16); and eight with t(8;21)
- 8 patients in CR2: Five with inv(16); and three with t(8;21)
- 4 patients with active disease: Two with inv(16); and two with t(8;21)
Survival outcomes
- After a median follow-up of 4.43 years, median overall survival (OS) for the entire cohort was 4.48 years, and the 4-year OS rate was 51%
- Relapse-free survival (RFS) and OS were comparable between patients with inv(16) and those with t(8;21)
- Survival was extremely poor in patients who relapsed and did not receive allo-HSCT, irrespective of the CBF-AML type (median OS: 0.6 years; 95% CI, 0.31–1.11 years), with no patients surviving beyond 2 years
- Allo-HSCT markedly improved survival in patients who relapsed, with a significantly better median OS (not reached), and a 4-year OS rate (53%; 95% CI, 30–94%)
- Subset analysis within patients receiving intensive treatment revealed that trisomy 22 in those with inv(16) was a favorable prognostic marker for RFS (Figure 2)
- Subtype of CBF, along with trisomy 8, patient age, and complex karyotype, had no effect on outcome
Figure 2. 4-year RFS for patients with trisomy 22 with inv(16) and patients with other CBF-AML subtypes*
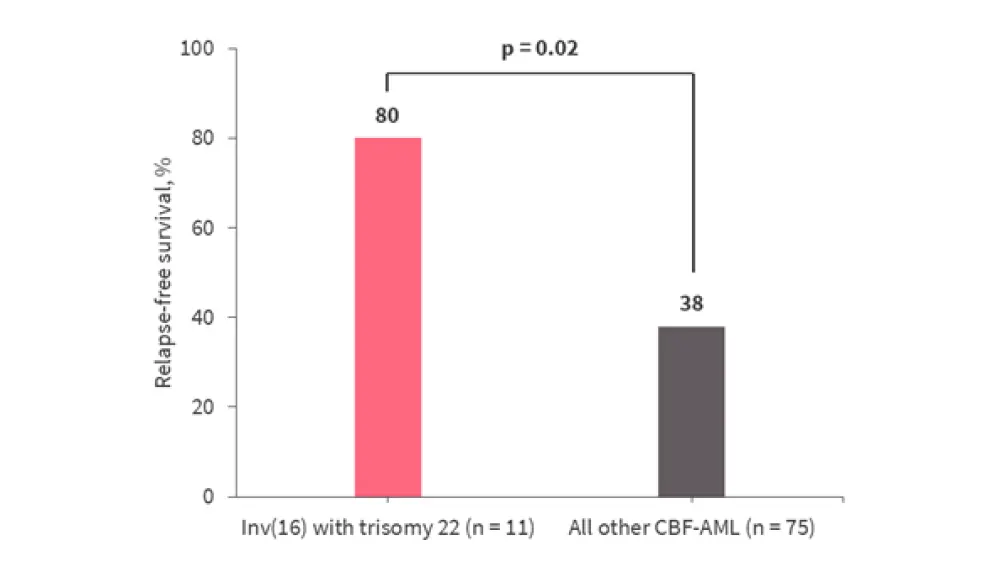
CBF-AML, core-binding factor acute myeloid leukemia.
*Data from Kayser et al.1
Conclusion
Results from the study1 demonstrate that patients with CBF-AML respond well to intensive chemotherapy, with high rates of CR irrespective of concurrent FLT3-ITD mutations. However, based on previously published data, patients with FLT3-ITD CBF-AML, excluding inv(16) with trisomy 22, demonstrated inferior outcomes compared to those with wildtype FLT3-ITD. The authors highlighted the 4-year OS rate of 51% observed in patients with FLT3-ITD CBF-AML who received intensive treatment, and compared this result to the 10-year OS rate of 58% reported in a previous study,2 conducted in patients with CBF-AML without the FLT3-ITD mutation. Thus, Kayser et al.1 recommend that the subgroup of patients with CBF-AML and FLT3-ITD should not be classified as ‘favorable risk’.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

