All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
The latest on trials of quizartinib from EHA 2023
In patients with acute myeloid leukemia (AML), FLT3-internal tandem duplication (ITD) mutations are associated with poor outcomes.1 Inhibition of FLT3 and other kinases may also improve outcomes in patients with AML with wild-type (wt) FLT3.2 Quizartinib is a selective type II FLT3 inhibitor that has been extensively reported on by the AML Hub.
Several presentations at the European Hematology Association (EHA) 2023 Hybrid Congress discussed results from clinical trials evaluating the efficacy and safety of quizartinib in patients with AML (Table 1). Below, we summarize the key findings from oral presentations by Schlenk,1 Montesinos,2 Knapper,3 and Bergua Burgues,4 and a poster presentation by De La Fuente.5
Table 1. Overview of studies presented at EHA 2023 involving quizartinib*
|
Trial |
Intervention |
Design |
Primary endpoint(s) |
|---|---|---|---|
|
QuANTUM-First NCT02668653 |
Quizartinib in addition to standard chemotherapy vs placebo in patients aged 18–75 years with ND FLT3-ITD-mutated AML |
Randomized phase III trial. Induction: Cytarabine + daunorubicin or idarubicin with quizartinib 40 mg/day or placebo. Consolidation: High-dose cytarabine with quizartinib 40 mg/day or placebo. Continuation: Quizartinib 60 mg/day vs placebo |
OS |
|
QUIWI |
Quizartinib in addition to standard chemotherapy vs placebo in patients aged 18–70 years with ND FLT3-ITD wt AML |
Randomized phase II trial. Induction: Cytarabine + idarubicin with Quizartinib 60 mg/day or placebo. Consolidation: Cytarabine with quizartinib 60 mg/day or placebo. Maintenance: Quizartinib 60 mg/day or placebo |
EFS |
|
NCRI AML18 NCT02272478 |
Several therapeutic agents including quizartinib in patients aged ≥60 years with AML or HR-MDS with >10% blasts |
Randomized phase III trial. Daunorubicin/cytarabine or daunorubicin/cytarabine + cladribine or FLAG-Ida or IDAC ± quizartinib† |
OS, CR+CRi, DoR, toxicity, and supportive care requirements |
|
VEN-A-QUI |
Triplet combinations including quizartinib in ND patients aged >70 years or unfit and >65 years |
Phase I/II trial. Ven + Aza + quizartinib 60 mg or Ven + low-dose cytarabine + quizartinib 40 mg |
Phase I: RP2D Phase II: CR/CRi |
|
MDA-AML-2018-06 |
Midostaurin in combination with intensive chemotherapy in patients aged ≥18 years with FLT3-mutated AML |
Retrospective study |
Safety and efficacy, comparison of results with the RATIFY and QuANTUM-First trials |
|
AML, acute myeloid leukemia; Aza, azacitidine; CR, complete remission; CRi, CR with incomplete count recovery; DoR, duration of response; EFS, event-free survival; FLAG-Ida, fludarabine, high-dose cytarabine, idarubicin, and granulocyte-colony stimulating factor; HR, higher risk; IDAC, intermediate-dose cytarabine; ITD, internal tandem duplication; MDS, myelodysplastic syndromes; ND, newly diagnosed; OS, overall survival; RP2D, recommended phase II dose; Ven, venetoclax; wt, wild-type. †Refers to the quizartinib portion of the NCRI AML18 trial only. |
|||
QuANTUM-First1
Study design and patient characteristics
The AML Hub has previously shared findings from the phase III QuANTUM-First trial (NCT02668653), showing that the addition of quizartinib to standard chemotherapy and up to 3 years of continuation therapy improved overall survival (OS) versus placebo in 539 patients aged 18–75 years with newly diagnosed (ND) FLT3-ITD AML (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.62–0.98; p = 0.032). At EHA 2023, Schlenk1 presented an analysis of the impact of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in first complete remission (CR1) and measurable residual disease (MRD) on outcomes in patients in this trial.
Key findings
- Multivariable regression analysis of OS with allo-HSCT in CR1 as a time-dependent variable and adjusted for FLT3-ITD variant allele frequency and gender revealed:
- Treatment with quizartinib was associated with improved OS versus placebo (HR, 0.770; 95% CI, 0.609–0.973; p = 0.0284).
- Allo-HSCT in CR1 was associated with improved OS versus no allo-HSCT in CR1 (HR, 0.424; 95% CI, 0.301–0.597; p < 0.0001).
- Post hoc analysis revealed longer OS in patients treated with quizartinib versus placebo, irrespective of MRD status pre-allo-HSCT; although the survival benefit was more pronounced in those who were MRD+ pre-allo-HSCT (n = 55; HR, 0.471; 95% CI, 0.174–1.275) than MRD− (n = 96; HR, 0.717; 95% CI, 0.332–1.547).
- No new safety signals were observed in patients who underwent allo-HSCT, with medical conditions observed in 83.3% and 74.7% of patients treated with quizartinib and placebo, respectively.
- The most commonly observed medical conditions in patients receiving quizartinib after protocol-specified allo-HSCT were stomatitis (23.5%), pyrexia (14.7%), diarrhea (12.7%), nausea (11.8%), cytomegalovirus infection (11.8%), and rash (11.8%).
This post hoc analysis of the QuANTUM-First trial showed that the survival benefit of quizartinib compared with placebo was independent of allo-HSCT in CR1 and pre-allo-HSCT MRD status.
QUIWI2
Study design and patient characteristics
The multicenter, randomized, double-blind, placebo-controlled, phase II QUIWI trial (NCT04107727), from the Programa de Estudio y Tratamiento de las Hemopatías Malignas (PETHEMA) group, evaluated the addition of quizartinib to standard chemotherapy versus placebo in adult patients aged 18–70 years with ND FLT3-ITD wt AML who had an Eastern Cooperative Oncology Group (ECOG) performance status <3. Patients were stratified by age (<60 years, ≥60 years) and randomized 2:1 to receive idarubicin + cytarabine and quizartinib or placebo (Figure 1).
Figure 1. QUIWI study overview*
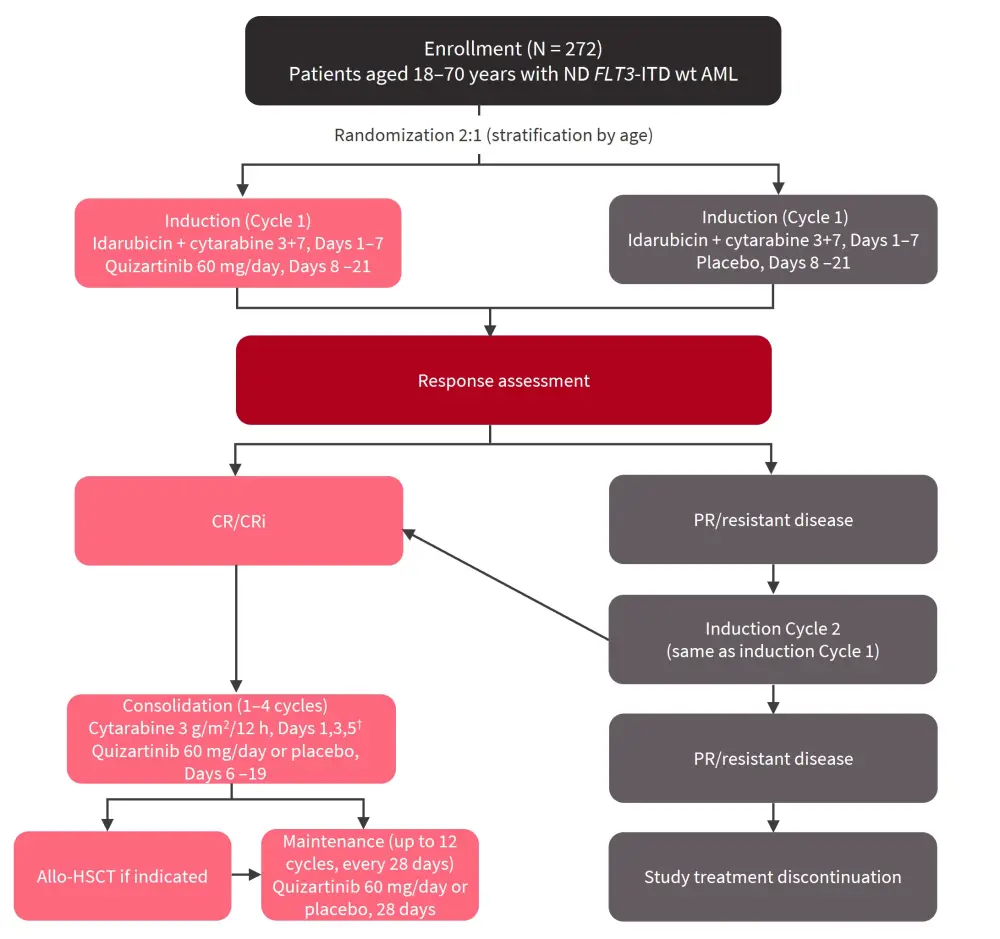
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; CR, complete remission; CRi, CR with incomplete count recovery; ITD, internal tandem duplication; ND, newly diagnosed; PR, partial remission; wt, wild-type.
*Adapted from Montesinos.2
†For patients ≥60 years: Cytarabine 1.5 g/m2/12 h, Days 1,3,5.
The primary endpoint was event-free survival (EFS), with secondary endpoints of OS, CR/composite CR, and safety. Relapse-free survival (RFS) and duration of CR were included as exploratory endpoints.
Montesinos2 presented results from a planned interim analysis 12 months after the last patient inclusion. Baseline characteristics were generally comparable between the two treatment arms, with a median age of 57.1 years and 58.5 years in the quizartinib and placebo arms, respectively. However, fewer patients were classified as favorable risk by the European LeukemiaNet (ELN) 2017 risk classification (19% vs 27%), more patients were intermediate risk (45% vs 27%), and fewer patients were adverse risk (36% vs 46%) in the quizartinib arm versus placebo arm (p = 0.01).
Key findings
- Response rates were similar between treatment arms (Figure 2).
Figure 2. Response rates with quizartinib were similar to placebo*
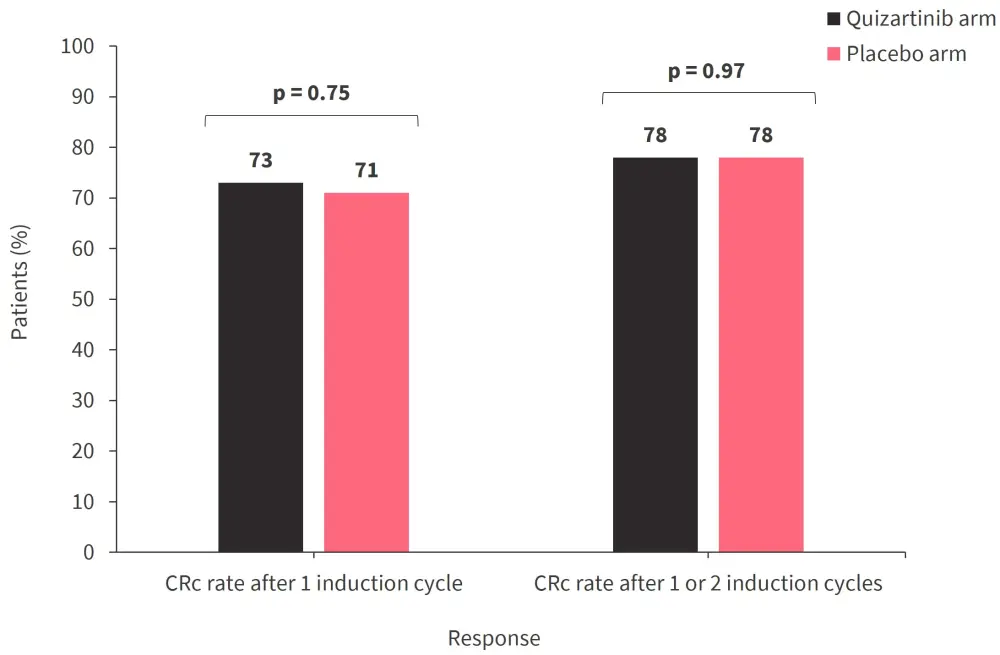
CRc, composite complete remission.
*Data from Montesinos.2
- Median EFS was numerically higher in the quizartinib arm (16.5 months) versus the placebo arm (10.6 months; HR, 0.741; 95% CI, 0.535–1.026; p = 0.059).
- Quizartinib was associated with an OS benefit (median OS, not reached [NR]) versus placebo (median OS, 20.2 months; HR, 0.569; 95% CI, 0.385–0.841; p = 0.004).
- Subgroup analysis for the OS benefit of quizartinib is detailed in Table 2.
- Quizartinib was also associated with a RFS benefit (median RFS, NR) versus placebo (median RFS, 18.6 months; HR, 0.631; 95% CI, 0.414–0.962; p = 0.031).
- No new safety signals were identified in the quizartinib arm.
Table 2. Subgroup analysis for OS benefit*
|
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; CI, confidence interval; ELN, European LeukemiaNet; HR, hazard ratio. |
||
|
Patient characteristic |
HR |
95% CI |
|---|---|---|
|
<60 years old |
0.595 |
0.3470–1.021 |
|
≥60 years old |
0.552 |
0.313–0.973 |
|
Underwent allo-HSCT |
0.610 |
0.267–1.396 |
|
Did not undergo allo-HSCT |
0.567 |
0.364–0.884 |
|
ELN 2017 favorable risk |
0.178 |
0.038–0.841 |
|
ELN 2017 intermediate risk |
0.353 |
0.162–0.770 |
|
ELN 2017 adverse risk |
0.908 |
0.554–1.487 |
Preliminary results from this study suggest that the addition of quizartinib to standard chemotherapy may improve survival outcomes versus placebo in patients with ND FLT3-ITD wt AML.
NCRI AML183
Study design and patient characteristics
The NCRI AML18 trial (NCT02272478) evaluated several therapeutic options in patients aged ≥60 years with AML or high-risk myelodysplastic syndromes with >10% blasts and ECOG performance status 0–2, between November 2014 and April 2022 (Figure 3).
Figure 3. NCRI AML18 study overview*
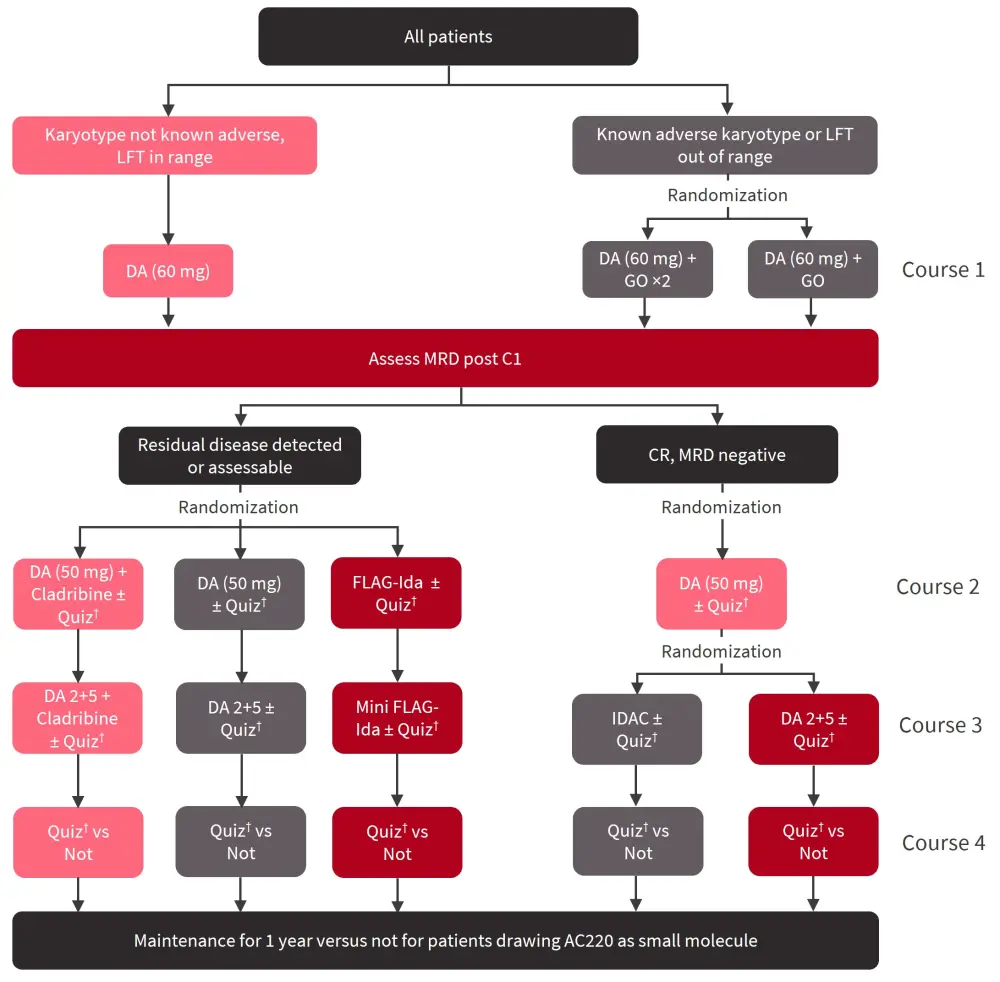
DA, daunorubicin/cytarabine; C1, Cycle 1; CR, complete remission; FLAG-Ida, fludarabine, high-dose cytarabine, idarubicin, and granulocyte-colony stimulating factor; GO, gemtuzumab ozogamicin; IDAC, intermediate-dose cytarabine; LFT, liver function tests; MRD, measurable residual disease; Quiz, quizartinib.
*Adapted from Knapper.3
†Further randomization 1:1 to long (n = 116) or short (n = 117) therapy.
In total, 464 patients were randomized 1:1 to receive quizartinib (n = 233) or not (n = 231), regardless of FLT3 status. Within the quizartinib cohort, patients were further randomized 1:1 to short (n = 117) or long (n = 116) therapy (which could include maintenance). The median follow-up was 54 months. Baseline patient characteristics were balanced between the quizartinib and no quizartinib arms and between the long and short quizartinib arms. However, there were slightly more patients with FLT3-ITD mutations in the short quizartinib arm (24%) when compared with the long quizartinib arm (15%).
Key findings
- OS was similar between patients who received quizartinib (35% alive) and those who did not (33% alive; HR, 1.035; 95% CI, 0.823–1.303; p = 0.769), including patients with FLT3 mutations (42% vs 23%; HR, 0.688; 95% CI, 0.428–1.106; p = 0.121).
- No improvement with quizartinib in RFS was seen either in the overall population (HR, 1.070; 95% CI, 0.855–1.341; p = 0.550) or in patients with FLT3 mutations (HR, 0.771; 95% CI, 0.493–1.206; p = 0.255).
- Short (43%) or long quizartinib treatment (27%) yielded similar OS rates in the overall population (rate ratio; 0.769; 95% CI, 0.556–1.062; p = 0.111); however, among patients with FLT3 mutations, short quizartinib treatment was associated with an OS benefit (55%) when compared with long quizartinib treatment (26%; HR, 0.450; 95% CI, 0.220–0.930; p = 0.027).
- The median number of cycles of quizartinib was 2 (range, 0–15) in the long quizartinib arm and 2 (range, 0–3) in the short quizartinib arm.
- The addition of quizartinib was generally well tolerated; however, there was a significant increase in cardiac toxicity when compared with the no quizartinib group (p = 0.023); toxicities were similar between the short and long quizartinib groups.
In older, previously untreated patients with AML, the addition of quizartinib was well tolerated but did not provide a survival benefit in this study. However, in patients with FLT3 mutations, survival was improved with short exposures to quizartinib, but not with longer durations of quizartinib as maintenance therapy.
VEN-A-QUI4
Study design and patient characteristics
The phase I/II VEN-A-QUI study investigated the safety and efficacy of venetoclax + azacitidine (Ven-Aza) or venetoclax + low-dose cytarabine in combination with quizartinib in ND patients aged >70 years or unfit patients >65 years. The primary objective of the phase I part of this study was to establish the recommended phase II dose, while the primary objective of the phase II part of this study was to compare CR/CRi rates.
The phase I part of this study included two parallel arms of azacitidine (n = 6) and low-dose cytarabine (n = 9), with dose escalating 3+3 cohorts to identify dose-limiting toxicities. The phase II section included a 1:1 randomization of Ven-Aza + quizartinib (n = 31) and Ven + low-dose cytarabine + quizartinib (n = 30). The phase II part of this study included 12 patients with FLT3-ITD mutations as an exploratory group.
Key findings
- Following the phase I part of this study, the recommended phase II dose of quizartinib was determined as 40 mg in the cytarabine arm and 60 mg in the azacitidine arm.
- In the phase II section of this study, response rates were similar between the arms (Table 3).
- OS was longer in patients with FLT3-ITD mutations (median OS, NR; range, 9.77–NR) versus FLT3 wt (median OS, 9.03 months; range, 4–NR; p = 0.042).
- EFS was improved in patients with FLT3-ITD mutations (median EFS, NR) versus FLT3 wt (median EFS; 4.33 months; range, 3.4–NR; p = 0.0098).
- Patients who received prior treatment with hypomethylating agents had a numerically shorter OS (median OS, 8.38 months; range, 4.0–NR) than patients who did not (median OS, NR; range, 9.5–NR; p = 0.06).
- In total, 68 out of 76 patients experienced ≥1 Grade 3 serious adverse events; there were no incidences of significant QT prolongation or cardiac arrhythmias.
Table 3. Phase II response rates and outcomes*
|
CR, complete remission; CRc, composite CR; EFS, event-free survival; MLFS; morphologic leukemia-free state; MRD−, measurable residual disease negative; NR, not reached; ns, not significant; OS, overall survival; PR, partial response. |
|||||||
|
Response, n (unless otherwise stated) |
Azacitidine arm |
Cytarabine arm |
p value |
||||
|---|---|---|---|---|---|---|---|
|
1st cycle |
4th cycle |
Best response |
1st cycle |
4th cycle |
Best response |
|
|
|
CRc (%) |
8 (26) |
8 (26) |
17 (55) |
9 (30) |
8 |
15 (50) |
1 |
|
CR MRD− (%) |
4 (13) |
5 |
10 (32) |
4 (13) |
5 |
4 (16) |
ns |
|
MLFS (%) |
1 (7) |
1 |
5 (16) |
4 (13) |
1 |
2 (7) |
ns |
|
PR (%) |
4 (13) |
2 |
3 (10) |
5 (13) |
1 |
5 (16) |
— |
|
Death in induction (%) |
7 (22) |
— |
— |
4 (13) |
— |
— |
— |
|
Stable disease/progression (%) |
3 (6) |
0 |
— |
5 (16) |
1 |
— |
— |
|
Median OS, months (range) |
14.47 (9.03–NR) |
9.27 (5.6–NR) |
0.4 |
||||
|
Median EFS, months (range) |
8.03 (3.8–NR) |
6.50 (2.97–NR) |
0.87 |
||||
The VEN-A-QUI study suggests that triplet combinations of Ven-Aza + quizartinib and venetoclax + low-dose cytarabine + quizartinib could be feasible for ND unfit patients with substantial venetoclax and quizartinib reduction. Final analyses from this study with longer follow-up will be carried out to fully assess the potential benefit of these combinations.
MDA-AML-2018-065
Study design and patient characteristics
The MDA-AML-2018-06 study was a multicenter, retrospective study that evaluated the safety and efficacy of midostaurin and compared results with the phase III RATIFY trial, which was previously covered by the AML Hub, and QuANTUM-First trial. Patients included were aged ≥18 years, had FLT3 mutated AML, and were treated with midostaurin in combination with intensive chemotherapy as a first-line therapy.
In total, 175 patients were included in this retrospective analysis, of which 133 had a FLT3-ITD mutation. The median age was 53 years (range, 18–76).
Key findings
In the overall population, the 2-year OS rate was 68%. Among patients with a FLT3-ITD mutation:
- Median follow-up was 13 months
- CR rate was 83.4%
- Median OS was not reached, and the 2-year OS rate was 65%
- 8.3% of patients experienced QT prolongation; there were no deaths associated with midostaurin
In this retrospective analysis, midostaurin plus intensive chemotherapy as a first-line therapy for patients with FLT3-ITD-mutated AML appeared to be superior to quizartinib, based on previously reported results from a phase III trial.
Conclusion
Several presentations at the EHA 2023 Hybrid Congress discussed results from analyses of quizartinib in patients with AML, with varying results between these studies. There were some key differences in patient characteristics and treatment received between these studies which must be considered when comparing the results.
The post hoc analysis from the QuANTUM-First trial demonstrated that the survival benefit of quizartinib versus placebo was independent of allo-HSCT in CR1 and MRD status.1 While the QuANTUM-First trial showed the benefit of quizartinib in patients with FLT3-ITD AML, results from the QUIWI trial suggest that quizartinib may also be beneficial in patients with FLT3-ITD wt AML.2 Conversely, in the NCRI AML18 trial, quizartinib was associated with a survival benefit in patients with FLT3 mutations but not in the overall population; however, it is important to note that the NCRI AML18 trial included an older population than in the QUIWI trial.3 The VEN-A-QUI trial suggests that triplet combinations including quizartinib may be beneficial in older and unfit patients, and this positive effect was most evident in patients with FLT3-ITD mutations.4 Finally, the MDA-AML-2018-06 study suggested improved outcomes with midostaurin versus quizartinib in patients with FLT3-ITD mutations; although this analysis was limited by its retrospective nature.5
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


