All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
The history and evolution of the role of venetoclax in the treatment of AML
Despite only being approved in the last few years, venetoclax has already become a standard of care for treating patients with acute myeloid leukemia (AML). In a talk during the 2nd National Cancer Research Institute (NCRI) AML Academy, Andrew Wei discussed the management of patients with AML and the role of venetoclax.1
Introduction
Bcl-2 is a pro-survival factor that was discovered in the late 1980s. Bcl-2 functions by preventing the activation of downstream molecules, Bax and Bak. Upon activation, Bax can form pores in the outer mitochondrial membrane, leading to the release of cytochrome c and triggering apoptosis. Like other pro-survival molecules, Bcl-2 blocks apoptosis through interaction with its BH3 domain. Knowledge of this structure was used to create venetoclax, a specific small molecular inhibitor of Bcl-2, which can increase apoptosis in tumor cells.
Venetoclax and AML
While these initial tests were promising, a phase I trial with venetoclax showed an overall response rate of only 19% and a duration of response of only a few months. It was known that other pro-survival molecules, such as MCL1, were upregulated in AML, however, no specific inhibitors were available at the time. Therefore, venetoclax was used in combination with the chemotherapy drugs that were available.
To investigate the efficacy of venetoclax in combination, two phase Ib/II studies were performed in patients with first-line AML, as shown in Table 1. It was clear from these trials that efficacy was far greater than had been achieved previously with cytotoxic agents alone. In elderly patients, survival beyond 2 years was rarely seen; however, with venetoclax combinations, a subset of these patients was able to achieve extended survival.
Table 1. Comparison of survival outcomes in patients treated with venetoclax combinations1
|
CR, complete response; CRi, complete response with incomplete hematological recovery; HMA, hypomethylating agent; LDAC, low-dose ara-c; OS, overall survival. |
||
|
|
Venetoclax with LDAC |
Venetoclax with HMA |
|---|---|---|
|
Median age |
74 |
74 |
|
CR/CRi (%) |
54 |
67 |
|
OS (months) |
10.1 |
17.5 |
Venetoclax was approved in 2018 by the U.S. Food and Drug Administration (FDA). On the basis of these previous results, two phase III trials, VIALE-A and VIALE-C, were initiated:
- VIALE-A compared venetoclax + azacitidine with azacitidine alone
- VIALE-C compared venetoclax + low-dose ara-c (LDAC) with LDAC alone
The survival outcomes for patients enrolled in these trials are shown in Table 2.
Table 2. Survival outcomes for VIALE-A and -C clinical trials in patients with first-line AML1
|
CR, complete response; CRi, complete response with incomplete hematological recovery; LDAC, low-dose ara-c; OS, overall survival; TRM, treatment-related mortality. |
||
|
VIALE-A |
Venetoclax + azacitidine |
Azacitidine + placebo |
|---|---|---|
|
CR/CRi (%) |
66 |
28 |
|
OS (months) |
14.7 |
9.6 |
|
30-day TRM (%) |
7 |
6 |
|
VIALE-C |
Venetoclax + LDAC |
LDAC + placebo |
|
CR/CRi (%) |
48 |
13 |
|
OS (months) |
8.4 |
4.1 |
|
30-day TRM (%) |
13 |
16 |
In both cases, overall survival (OS) was improved following treatment with the venetoclax combination compared with the chemotherapy + placebo group. However, only after post hoc analysis was the complete response (CR) rate significant in the VIALE-C trial. These trials confirmed venetoclax as a new standard of care for patients, particularly elderly patients with AML.
To use venetoclax effectively, treatment-associated adverse events, such as myelosuppression, must be balanced with efficacy. As venetoclax affects leukemic and hematopoietic stem cells alike, treatment can cause anemia and neutropenia. As a result, if patients achieve CR but are severely neutropenic after Day 28 of treatment, therapy interruption for up to 2 weeks is advised. Granulocyte-colony stimulating factor can also be beneficial. Once blood counts have recovered, the next cycle can proceed.
It is also advisable in patients who struggle to recover normal bone marrow function after treatment cycles, to reduce venetoclax duration and reduce the dose of azacitidine along with allogeneic hematopoietic stem cell transplant (allo-HSCT) in patients who have undergone clonal hematopoiesis.
Resistance to venetoclax and associated mutations
Monitoring of patients for the emergence of resistant clones during treatment failure is important. Andrew Wei’s group investigated what mutations are commonly seen in response to venetoclax therapy. Three different patient groups were examined: patients with a durable remission (> 12 months), patients with adaptive resistance (remission followed by relapse), and patients with primary resistance.
- NPM1 and IDH1/2 mutations were the most frequent changes in patients that achieved a durable remission.
- TP53 or FLT3 mutations were more common in patients that relapsed or were primary refractory.
Patients with an NPM1 mutation show increased susceptibility to venetoclax treatment. In the VIALE-A and -C trials within this subset, 67% and 79% of patients achieved CR/complete response with incomplete hematological recovery (CRi), respectively. FLT3-ITD, on the other hand, was associated with resistance to treatment, and some patients even developed novel FLT3-ITD mutations during therapy. Andrew Wei reported on five cases in which a strong selection bias was also demonstrated for the development of TP53 mutations following venetoclax treatment.
Current role of venetoclax in patients with AML
In addition to the treatment recommendations listed in Table 3, a triplet therapy has also been proposed using azacitidine + venetoclax + FLT3-ITD inhibitor for first-line patients, but the degree of myelosuppression caused has yet to be examined.
Table 3. Venetoclax combination treatment recommendations for AML subtypes from the VIALE trials1
|
FLT3i, fms-like tyrosine kinase 3 inhibitor; LDAC, low-dose ara-c; R/R, relapsed/refractory. |
||||
|
Mutation |
IDH |
NPM1 |
FLT3-ITD |
TP53 |
|---|---|---|---|---|
|
Treatment |
Venetoclax + azacitidine |
Venetoclax + azacitidine or venetoclax + LDAC |
First line: Venetoclax + azacitidine or FLT3i + azacitidine R/R: FLT3i ± venetoclax |
Venetoclax + azacitidine |
In the VIALE-A trial, patients with hypomethylating agent failure were excluded. Historically, this group required allo-HSCT as they do not respond favorably to chemotherapy. Even in this group, azacitidine + venetoclax can have a positive impact on survival. A recent study showed an increase in overall response rate from 8% to 40% and an increase in OS from 5.5 months to 12 months compared with venetoclax monotherapy.
The CAVEAT study (ACTRN12616000445471) investigated the use of intensified therapy in patients (≥ 65 years) with AML, including patients with TP53 mutations. Venetoclax + cytarabine + idarubicin was used as the induction regimen in these elderly patients, followed by four rounds of consolidation and seven cycles of maintenance therapy. The use of each agent was staggered to reduce the risk of tumor lysis syndrome. Patients with de novo AML in this group demonstrated a good OS, whereas secondary AML still fared poorly. The median OS in the de novo group was 31.3 months compared with only 6.1 months in the secondary group. The response rate was still poor in patients with TP53 mutations, with none achieving CR and an OS of only 3.6 months.
Potential future therapies for this subset of patients include the following:
- Azacitidine + APR-246, which achieved a CR/CRi of 80% in a recent study
- Azacitidine + magrolimab, which reached a CR/CRi of 75% in a recent study
- Allo-HSCT is also a valuable treatment option
Future of AML therapy
A potential strategy could be using multi-agent treatment schemes coupled with molecular monitoring to identify the development of new clones. To examine this idea further, the Investigating Novel Therapy to target Early Relapse and Clonal Evolution as Pre-emptive Therapy in AML (INTERCEPT) trial was set up, and the study schema is shown in Figure 1. Patients enter this study after first reaching measurable residual disease negativity and are monitored for relapse. Therapies are rotated depending on the results of molecular monitoring. If a patient has none of the mutations listed, they are treated with the best available therapy.
Figure 1. INTERCEPT study design1
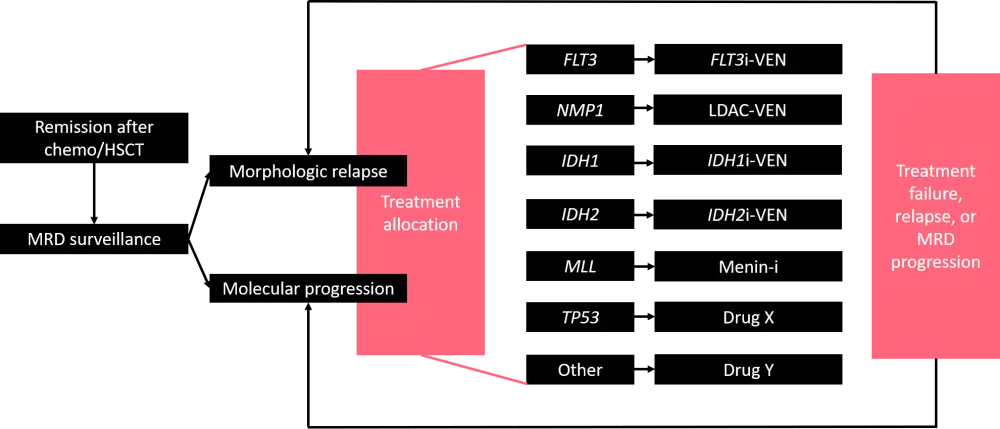
FLT3i, fms-like tyrosine kinase 3 inhibitor; LDAC, low-dose ara-c; MRD, measurable residual disease; HSCT, hematopoietic stem cell transplant; VEN, venetoclax.
Conclusion
Over the last 30 years, great progress has been made following the discovery of Bcl-2 and the development of venetoclax. This small molecule inhibitor has changed the landscape of AML treatment and improved survival outcomes in many patients with AML. In the future, novel therapies may be developed but venetoclax may still play a role as part of new triplet treatments. The importance of adapting therapies according to molecular progression within a patient has also been highlighted and may become integral to future treatment regimens.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

