All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Targeting the menin–KMT2A complex in AML: Current status of emerging therapies
Do you know... For which population is bleximenib being investigated as a monotherapy in the cAMeLot-1 clinical trial?
NPM1 mutations are present in approximately 25–30% of adults diagnosed with acute myeloid leukemia (AML), and KMT2A rearrangements occur in approximately 5–10% of patients diagnosed with AML or acute lymphoblastic leukemia.1 Outcomes are poor for these populations; patients with relapsed NPM1-mutated (NPM1m) AML have a median overall survival (OS) of 6.1 months, while in those with KMT2A-rearranged (KMT2Ar) AML it is just 2.4 months.1
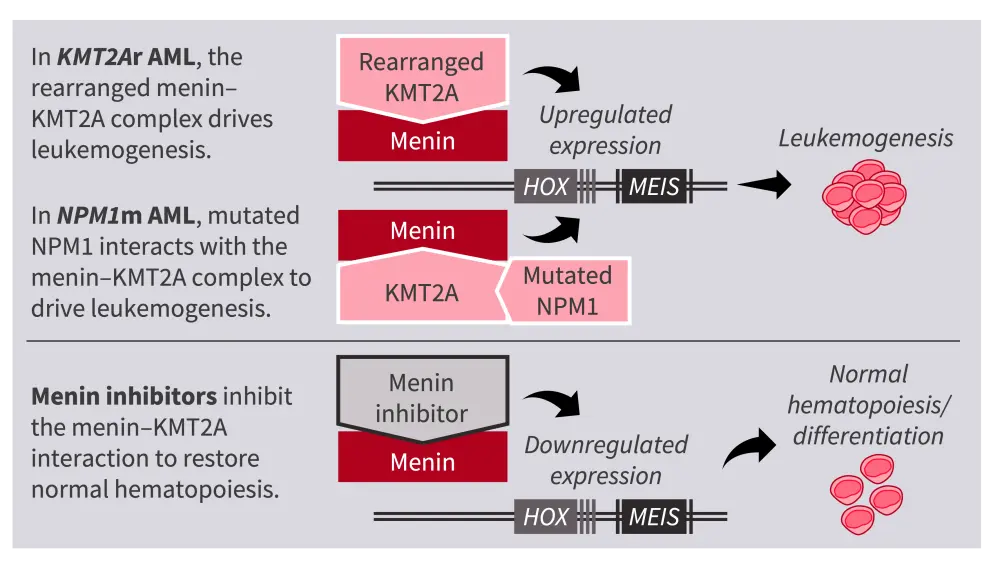
In nonleukemic cells, the KMT2A protein and menin interact to regulate HOX/MEIS gene transcription and maintain normal hematopoiesis. In KMT2Ar and NPM1m AML, the abnormal complex of rearranged KMT2A protein and menin, or an abnormal interaction between mutated NPM1 protein and the menin–KMT2A complex, results in upregulated expression of HOX and MEIS, which in turn drives leukemogenesis.1,2 Thus, the menin–KMT2Ar complex is a promising therapeutic target in AML; menin inhibitors act on the menin–KMT2A complex, resulting in downregulated expression of HOX and MEIS, and restoration of normal hematopoiesis (Figure 1).1,2
Figure 1. Mechanism of action of menin inhibitors in NPM1m or KMT2Ar AML1,2
Several menin inhibitors are in development for the treatment of NPM1m or KMT2Ar AML, and revumenib has been approved by the U.S. Food and Drug Administration (FDA) for patients aged ≥1 year with KMT2Ar relapsed/refractory (R/R) acute leukemia.3 Here, we summarize the current status of menin inhibitors in development, and provide an overview of key findings from clinical trials thus far.
Bleximenib
Bleximenib is a potent, selective menin inhibitor currently being investigated in phase I, I/II, and III clinical trials, both as a monotherapy and in combination strategies for the treatment of NPM1m/KMT2Ar AML, as summarized in Table 1.4–7
Table 1. Ongoing clinical trials investigating bleximenib in AML
| Trial | Intervention | Indication | Primary outcomes |
| ALE1002 (phase I; NCT05453903)4,5 | Bleximenib with Ven + Aza or 7+3 | ND or R/R AML with NPM1m or KMT2Ar AML | Safety; RP2D |
| cAMeLot-1 (phase I/II; NCT04811560)6 | Bleximenib monotherapy | R/R AML with NPM1m or KMT2Ar AML | Safety; RP2D; CR/CRh |
| cAMeLot-2 (phase III; NCT06852222)7 | Bleximenib with Ven + Aza | ND AML with NPM1m or KMT2Ar AML | OS; CR |
7+3, cytarabine + anthracycline; AML, acute myeloid leukemia; Aza, azacitidine; CR, complete remission; CRh, CR with partial hematologic recovery; KMT2Ar, KMT2A-rearranged; ND, newly diagnosed; NPM1m, NPM1-mutated; OS, overall survival; RP2D, recommended phase II dose; R/R, relapsed/refractory; Ven, venetoclax.
The ALE1002 trial
The ongoing phase I ALE1002 trial (NCT05453903) is investigating the safety and efficacy of bleximenib in combination with venetoclax (Ven) + azacitidine (Aza) in patients with NPM1m or KMT2Ar AML who are newly diagnosed or R/R, or with a 7+3 regimen in those who are newly diagnosed.4,5 Initial findings have been previously reported on the AML Hub.8
An updated analysis of the ALE1002 trial, aiming to determine the recommended phase II dose (RP2D) of bleximenib with Ven + Aza in ND or R/R patients with NPM1m or KMT2Ar AML (N = 125), was presented at the European Hematology Association (EHA) 2025 Congress, June 12–15, 2025, Milan, IT.5
Key findings5
- Results from the dose selection phase of the trial determined 100 mg twice daily (BID) as the RP2D for bleximenib in combination with Ven + Aza in this population.
- High response rates were observed at this dose in both newly diagnosed and R/R cohorts.
- In patients with newly diagnosed AML (n = 20) the overall response rate (ORR) was 90.0%, and the composite complete response (cCR) was 75.0%.
- In patients with R/R AML (n = 22), the ORR was 81.8% and the cCR was 59.1%.
- The median time to first response was 22 days (range, 18–57) in patients with newly diagnosed AML and 23 days (range, 18–50) in those with R/R AML.
- The safety profile was consistent with that of Ven + Aza.
- Grade ≥3 treatment-emergent adverse events (TEAEs) were observed in 97% of all patients treated. The most common hematological TEAEs (occurring in ≥20% of patients) were thrombocytopenia (55%), anemia (54%), neutropenia (50%), febrile neutropenia (39%), and leukopenia (23%).
- In patients who achieved CR/CRh at the RP2D (newly diagnosed, n = 13; R/R, n = 9), the median time to platelet recovery was 29 days in newly diagnosed patients and 38 days in R/R patients, and the median time to neutrophil recovery was 31 days and 32 days, respectively.
- Grade ≥3 differentiation syndrome (DS) occurred in 4% of patients, no corrected QT interval (QTc) prolongation signals were identified, and no dose interruptions or reductions occurred.
The cAMeLot-1 trial
The ongoing phase I/II cAMeLot-1 trial (NCT04811560) is looking at the safety and efficacy of bleximenib monotherapy in patients with R/R NPM1m or KMT2Ar acute leukemia.6,9 Initial findings from the dose-finding phase I part of the trial have been reported previously,9 and are summarized here.
Key findings9
- At the data cut-off of October 2024, 146 patients had received bleximenib monotherapy treatment.
- In the intention-to-treat (ITT) efficacy population, the ORR was 36.4% with bleximenib 45 mg BID (n = 11), 47.6% with 90/100 mg BID, and 55.0% (n = 21) with 150 mg BID (n = 20). The cCR rates were 18.2%, 33.3%, and 40.0%, respectively.
- Responses were comparable in patients with NPM1m and KMT2Ar acute leukemia.
- In the 90/100 mg BID cohort, the median time to first response was 1.4 months (range, 0.9–4.7).
- Bleximenib demonstrated a tolerable safety profile in the ITT safety population (N = 146).
- Grade ≥3 treatment-related adverse events (TRAEs) occurred in 22.6% of patients receiving 90/100 mg bleximenib BID (n = 31), and 36.4% of patients receiving 150 mg BID (n = 33). The most common Grade ≥3 TRAEs with 90/100 mg bleximenib BID were thrombocytopenia (9.7%), DS (6.5%), and neutropenia (3.2%).
- The majority of DS events reported were low grade, and no QTc prolongation has been observed.
- Dose interruptions/reductions were more frequent with the 150 mg BID dose than with the 90/100 mg BID dose.
- Based on these data, 100 mg BID, with an initial 50 mg step-up dose for 14 days, was determined as the RP2D.
- The phase II part of the cAMeLot-1 trial, to further investigate bleximenib at the RP2D in this population, is ongoing.
The cAMeLot-2 trial7
The phase III cAMeLot-2 trial (NCT06852222) is evaluating the safety and efficacy of bleximenib in combination with Ven + Aza vs placebo with Ven + Aza, in patients with newly diagnosed NPM1m or KMT2Ar AML who are ineligible for intensive chemotherapy. The primary endpoints are CR and OS. This trial is currently recruiting patients, with an estimated enrollment of 600 patients. The estimated primary completion date is June 2029.
Revumenib
Revumenib is a first-in-class, potent, selective menin inhibitor currently being investigated as a monotherapy and in combination strategies for the treatment of NPM1m/KMT2Ar AML in phase I, I/II, and III clinical trials, summarized in Table 2.10–18
Table 2. Ongoing clinical trials investigating revumenib in AML
| Trial | Intervention | Indication | Primary outcomes |
| INTERCEPT (phase I; ACTRN12622000582752)10 | Revumenib monotherapy | NPM1m or KMT2Ar AML in CR1 or CR2 | MRD clearance |
| NCT05886049 (phase I)11 | Revumenib with 7+3 | ND NPM1m or KMT2Ar AML | MTD; RP2D |
| SAVE (phase I/II; NCT05360160)12 | Revumenib with Ven + HMA | ND ineligible for intensive chemotherapy or R/R NPM1m or KMT2Ar AML or MPAL | Safety; tolerability; RP2D |
| Beat AML (phase Ib; NCT03013998)13 | Revumenib with Ven + Aza | ND NPM1m or KMT2Ar AML | RP2D; safety |
| EVOLVE-2 (phase III; NCT06652438)14 | Revumenib with Ven + Aza | ND NPM1m or KMT2Ar AML | OS |
| AUGMENT-101 (phase I/II; NCT04065399)15–17 | Revumenib monotherapy | R/R NPM1m or KMT2Ar AML, ALL, or MPAL | CR+CRh rate; safety; tolerability |
| AUGMENT-102 (phase I; NCT05326516)18 | Revumenib with chemotherapy | R/R NPM1m or KMT2Ar AML, ALL, or ALAL | Safety; tolerability |
7+3, cytarabine + anthracycline; ALAL, acute leukemias of ambiguous lineage; AML, acute myeloid leukemia; Aza, azacitidine; CR, complete remission; CRh, CR with partial hematologic recovery; CRi, CR with incomplete hematologic recovery; HMA, hypomethylating agent; KMT2Ar, KMT2A-rearranged; MLFS, morphologic leukemia-free state; MPAL, mixed phenotype acute leukemia; MRD, measurable residual disease; MTD, maximum tolerated dose; ND, newly diagnosed; NPM1m, NPM1-mutated; OS, overall survival; RP2D, recommended phase II dose; R/R, relapsed/refractory; Ven, venetoclax.
AUGMENT-101
The phase I/II AUGMENT-101 trial (NCT04065399) is evaluating the safety and efficacy of revumenib monotherapy in patients with R/R NPM1m or KMT2Ar AML. Findings from both the NPM1m and KMT2Ar cohorts have been reported previously; data from updated analyses presented at EHA 2025 are summarized here.15–19
Key findings
- In the updated analysis in patients with R/R NPM1m AML (n = 84), revumenib demonstrated clinically meaningful responses and a tolerable safety profile, consistent with previous reports.16
- In the efficacy-evaluable population of patients with R/R NPM1m AML (n = 77), the ORR was 48.1% and the CR+CRh rate was 26.0%. Among patients with CR+CRh, the median OS was 23.3 months (95% CI, 7.2–not reached).
- Grade ≥3 TEAEs occurred in 91.7% of patients; the most common were febrile neutropenia (33.3%), anemia (25.0%), QTc prolongation (22.6%), decreased platelet count (16.7%), and sepsis (15.5%).
- Grade ≥3 TRAEs occurred in 59.5% of patients; discontinuation due to TRAEs occurred in 4.8% of patients.
- The FDA has granted priority review to the supplemental new drug application for revumenib in R/R NPM1m AML, with a Prescription Drug User Fee Act target action date of October 25, 2025.19
- Clinically meaningful responses were also demonstrated in patients with R/R KMT2Ar AML (n = 116).17
- The safety profile was manageable and consistent with that previously reported. Grade ≥3 TEAEs occurred in 91.4% of patients; the most common were febrile neutropenia (38.8%), anemia (19.8%), and decreased platelet count (16.4%).
- Grade ≥3 TRAEs occurred in 54.3% of patients. There were no discontinuations due to cytopenias, DS, or QTc prolongation.
- In the efficacy-evaluable population with R/R KMT2Ar AML (n = 97), the ORR was 63.9% and the CR+CRh rate was 22.7%. Among patients with CR+CRh, 34% were able to proceed to hematopoietic stem cell transplantation.
- Based on the findings from this analysis, revumenib was approved by the FDA for the treatment of patients with R/R KMT2Ar AML.3
Ziftomenib
Ziftomenib is currently being investigated in phase I, I/II, and III clinical trials for the treatment of NPM1m/KMT2Ar AML, both as monotherapy and in combination strategies. We summarize these trials in Table 3.20–23
Table 3. Ongoing clinical trials investigating ziftomenib in AML
| Trial | Intervention | Indication | Primary outcomes |
| KOMET-001 (phase I/II; NCT04067336)20 | Ziftomenib monotherapy | R/R NPM1m or KMT2Ar AML or ALL | Safety; MTD; RP2D; CR/CRh |
| KOMET-007 (phase I; NCT05735184)21 | Ziftomenib with Ven + Aza or 7+3 | ND or R/R NPM1m or KMT2Ar AML in CR1 or CR2 | Safety; tolerability; CR rate |
| KOMET-008 (phase I; NCT06001788)22 | Ziftomenib with gilteritinib; FLAG-IDA; LDAC | R/R NPM1m or KMT2Ar AML | Safety; tolerability |
| KOMET-017 (phase III; NCT07007312)23 | Ziftomenib with Ven + Aza or 7+3 | ND NPM1m or KMT2Ar AML | OS; CR; EFS |
7+3, cytarabine + anthracycline; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; Aza, azacitidine; CR, complete remission; CRh, CR with partial hematologic recovery; EFS, event-free survival; FLAG-IDA, fludarabine, cytarabine, granulocyte colony-stimulating factor, idarubicin; KMT2Ar, KMT2A-rearranged; LDAC, low-dose cytarabine; MTD, maximum tolerated dose; ND, newly diagnosed; NPM1m, NPM1-mutated; OS, overall survival; R/R, relapsed/refractory; Ven, venetoclax.
KOMET-001
The ongoing pivotal phase I/II KOMET-001 trial (NCT04067336) is evaluating the safety and efficacy of ziftomenib monotherapy in patients with R/R NPM1m or KMT2Ar AML. Safety, tolerability, and preliminary clinical activity with ziftomenib in this trial have been reported previously. Clinical activity and safety data from the phase II portion of KOMET-001 were presented at EHA 2025 and are summarized here.
Key findings24
- Patients with R/R NPM1m AML treated with 600 mg ziftomenib once daily (n = 92) were included in the registration-enabling phase II analysis.
- The phase II primary endpoint was met in this population, with a 23% CR/CRh rate vs 12% historical control rate (p = 0.0058).
- The ORR was 33%, and MRD negativity was observed in 63% of responders.
- Ziftomenib demonstrated a tolerable safety profile, consistent with previous reports.
- Grade ≥3 TEAEs occurred in 93% of patients; the most common were febrile neutropenia (26%), anemia (20%), thrombocytopenia (20%), and DS (15%).
- Grade ≥3 TRAEs occurred in 40% of patients. No significant QTc prolongation was observed, and DS was successfully managed with mitigation strategies.
Discontinuation due to TRAEs occurred in 3% of patients.
Enzomenib and balomenib
Enzomenib is currently being evaluated in a phase I/II trial in patients with R/R NPM1m or KMT2Ar AML or acute lymphoblastic leukemia, as summarized in Table 4.25
Table 4. Ongoing clinical trials investigating enzomenib or balomenib in AML
| Trial | Intervention | Indication | Primary outcomes |
| DSP-5336-101 (phase I/II; NCT04988555)25 | Enzomenib monotherapy | R/R NPM1m or KMT2Ar AML or ALL | Safety; tolerability; RP2D; efficacy |
| NCT06780124 (phase I) 26 | Balomenib monotherapy | Healthy volunteers | Pharmacokinetics |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; KMT2Ar, KMT2A-rearranged; NPM1m, NPM1-mutated; RP2D, recommended phase II dose; R/R, relapsed/refractory.
DSP-5336-101
The phase I/II dose escalation and expansion DSP-5336-101 trial (NCT04988555) of enzomenib in patients with R/R NPM1m or KMT2Ar AML or acute lymphoblastic leukemia is ongoing. Initial findings have been reported previously on the AML Hub and are summarized here.27
Key findings27
- In two optimized dose cohorts of 200 mg enzomenib twice daily and 300 mg twice daily, the total ORR was 58.8% in patients with KMT2Ar acute leukemia, and 65.2% in patients with NPM1m acute leukemia.
- The CR/CRh was 47.1% in patients with NPM1m acute leukemia and 30.4% in patients with KMT2Ar acute leukemia.
- Enzomenib was well tolerated across the dose levels assessed (40 mg to 400 mg twice daily).
- The most common Grade ≥3 TEAEs were sepsis (25.0%), febrile neutropenia (23.8%), reduced platelet count (21.4%), anemia (16.7%), pneumonia (16.7%), and reduced neutrophil count (16.7%).
- No dose-limiting toxicity, treatment-related deaths, or discontinuations were observed.
- DS occurred in 10.7% of patients, and QTc prolongation occurred in 1% of patients.
- Additional combination cohorts have been initiated, and dose optimization is ongoing to determine the RP2D for the phase II expansion portion.
Balomenib
Balomenib has demonstrated efficacy and a manageable safety profile in a preclinical setting,28 and is currently being evaluated in healthy volunteers in a phase I, single- and multiple-dose escalation clinical trial, as summarized in Table 4.26
Conclusion
Abnormal interactions between the product of mutated NPM1 or of rearranged KMT2A and menin drive leukemogenesis in NPM1m and KMT2Ar AML, and thus represent a promising therapeutic target in AML.1
The menin inhibitors summarized above have demonstrated favorable initial safety and efficacy data in patients with newly diagnosed and R/R AML, in both NPM1m and KMT2Ar populations. Revumenib has been approved by the FDA for patients with R/R KMT2Ar AML,3 and the supplemental new drug application for revumenib in patients with R/R NPM1m AML has been granted priority review.19 Bleximenib was granted orphan drug designation by the FDA in October 2024 for the treatment of acute myeloid leukemia.29 Promising data have been observed with other agents in this emerging class of agents, and clinical trials to further assess their therapeutic value, as both monotherapies and in combination regimens, are ongoing.
This educational resource is independently supported by Johnson & Johnson. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



.webp&w=3840&q=75)