All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Survival outcomes with midostaurin in patients with FLT3 mutated AML: Retrospective cohort from the AML-12 trial
Do you know... Midostaurin combined with intensive chemotherapy is a standard of care in patients with FLT3-mutated AML. Which outcome has been shown to have no difference with midostaurin exposure in patients with FLT3-mutated AML?
FMS-like tyrosine kinase 3 (FLT3) is a frequently occurring gene mutation in patients with acute myeloid leukemia (AML).1 The most common mutations of FLT3 include internal tandem duplication (FLT3-ITD) and point mutations of the tyrosine kinase 2 domain (FLT3-TKD). FLT3 mutations (FLT3mut) and particularly FLT3-ITD have a strong adverse impact on patients with AML, consequently making allogeneic hematopoietic stem cell transplantation (allo-HSCT) the recommended choice for post-remission treatment in fit patients with AML. However, with the potential benefit of FLT3 inhibitors, the indication for allo-HSCT in patients with FLT3-mutated AML may need to be redefined.1
Midostaurin is a multikinase, first generation, type 2 inhibitor with inhibitory effects over various protein kinases, approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for treating adult patients with FLT3-mutated AML.1 The RATIFY phase III trial, previously covered by the AML Hub, has demonstrated a significant improvement in overall survival (OS) and event-free survival (EFS) with midostaurin but was restricted to patients aged ≤59 years. Here we summarize a retrospective cohort study by Oñate et al.1 published in the Blood Cancer Journal, investigating the survival outcomes with midostaurin in fit, older patients with FLT3-mutated AML.
Study design
This was a retrospective cohort study in patients enrolled in the multicenter, open-label AML-12 phase II trial (NCT04687098). Patients were aged 18−70 years, diagnosed with de novo AML, and eligible for intensive chemotherapy within the AML-12 trial. Patients treated between 2012 and 2015 formed the early cohort and those treated between 2016 and 2020 formed the late cohort (Figure 1). All patients received the same chemotherapy except patients in the late cohort who received midostaurin since 2016.
Outcomes were defined according to the European LeukemiaNet (ELN) classification and included:
- Complete remission (CR) or CR without hematologic recovery
- OS
- EFS
- Cumulative incidence of relapse (CIR)
Figure 1. Study design*
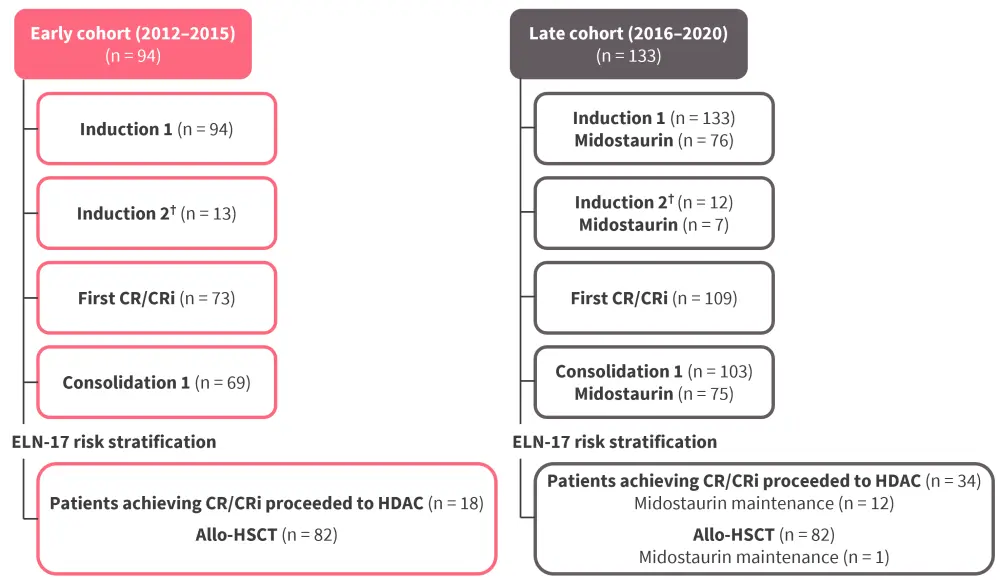
allo-HSCT, allogeneic hematopoietic stem cell transplantation; CR, complete remission; CRi, complete remission without hematologic recovery; ELN-17, European LeukemiaNet 2017; HDAC, high dose cytarabine.
*Adapted from Oñate, et al.1
†Only patients achieving partial response after first induction.
Results
Baseline characteristics
A total of 227 patients, 94 in the early cohort and 133 in the late cohort, were included. The median age was 54 years and 55 years in the early and late cohorts, respectively. The median follow-up was 42 months and selected baseline characteristics are shown in Table 1.
Table 1. Baseline characteristics*
|
allo-HSCT, allogeneic hematopoietic stem cell transplantation; BM, bone marrow; CR, complete remission; CR1, first complete remission, CR2, second complete remission; ECOG, Eastern Cooperative Oncology Group; ELN-17, European LeukemiaNet 2017; mut, mutation; WBC, white blood count; WT, wild type. |
||
|
Characteristics, % (unless otherwise stated) |
Early cohort |
Late cohort |
|---|---|---|
|
Age |
|
|
|
<60 years |
69 |
65 |
|
≥60 years |
31 |
35 |
|
Sex, female/male |
55/45 |
56/44 |
|
ECOG 0–1 |
82 |
77 |
|
Median WBC, x 109/L, (range) |
53 (1.6–314) |
45 (0.42–395) |
|
Median % of BM blasts (range) |
80 (21–100) |
80 (21–100) |
|
FLT3 mutations |
|
|
|
FLT3-ITD† |
98 |
75 |
|
Low ratio |
36 |
31 |
|
High ratio |
64 |
69 |
|
ELN-17 risk category |
|
|
|
Favorable |
32 |
34 |
|
NPM1mut/FLT3low |
83 |
56 |
|
NPM1mut/FLT3-TKD‡ |
3 |
36 |
|
Intermediate |
40 |
43 |
|
NPM1mut/FLT3high |
90 |
83 |
|
NPM1wt/FLT3low |
8 |
9 |
|
NPM1wt/FLT3-TKD |
3 |
9 |
|
Adverse |
28 |
23 |
|
NPM1wt/FLT3high |
89 |
77 |
|
CR rate |
78 |
82 |
|
Allo-HSCT |
56 |
62 |
|
CR1 |
41 |
48 |
|
CR2 |
6 |
7 |
|
Active disease |
5 |
5 |
Midostaurin and FLT3 mutation
The response rates in the early and late cohorts were similar (78% vs 82%) following induction therapy. At a median time of 3.5 months from CR to transplant, early relapse occurred in 9 and 5 patients in the early and late cohorts, respectively (p = 0.089).
- Patients achieving first CR in the early versus late cohorts showed an allo-HSCT rate of 64% and 68%, respectively.
- The presence of minimal residual disease (MRD) after first consolidation therapy was associated with higher 2-year relapse risk (p = 0.021) and worse survival (p = 0.055) in the overall cohort.
- There was no significant association between MRD negativity and the cohort time period or midostaurin exposure.
- Overall, patients in the late cohort showed improved outcomes compared with those in the early cohort (Figure 2).
Figure 2. Outcomes in the early versus late cohorts*
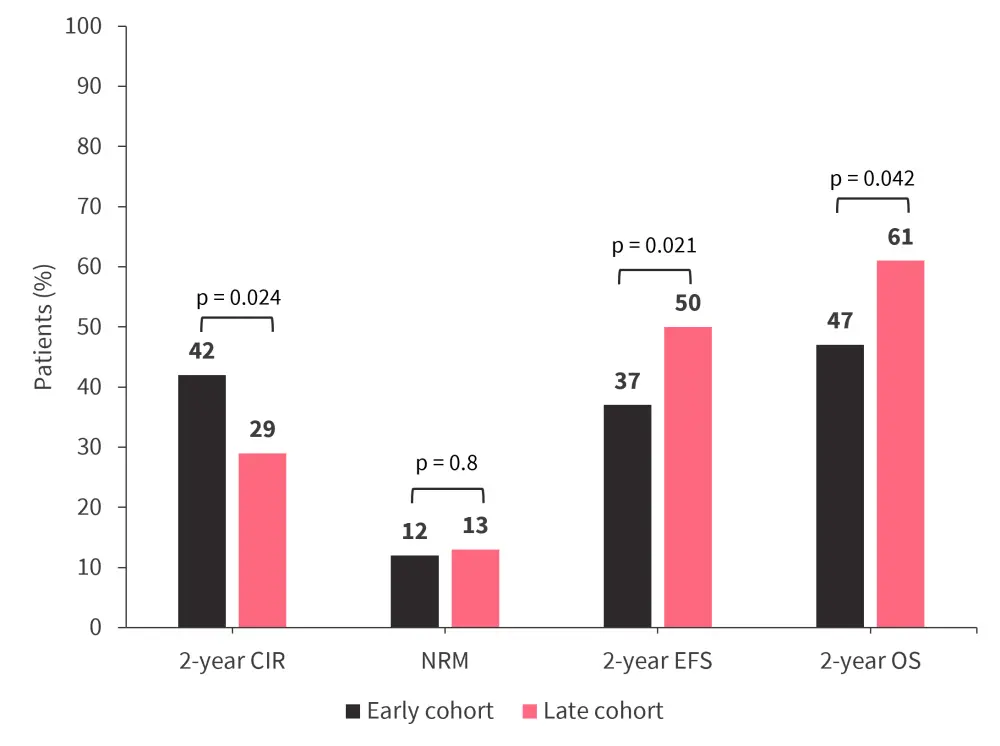
CIR, cumulative incidence of relapse; EFS, event-free survival; NRM, non-relapse mortality; OS, overall survival.
*Data from Oñate, et al.1
The univariate analysis in all patients with FLT3-mutated AML showed that leucocyte count at diagnosis, treatment period, ELN-17 FLT3 categories, and the presence of NPM1 mutation (NPM1mut) had a significant impact on the OS.
- The administration of midostaurin significantly improved OS in the whole cohort (hazard ratio [HR], 0.62; p = 0.024).
- The 2-year relapse rate was decreased (40% vs 28%; p = 0.034) and 2-year OS was improved (49% vs 65%; p = 0.023) in midostaurin-naïve and -exposed patients, respectively.
- Of note, patients in the late cohort who were midostaurin-naïve showed similar survival outcomes to those in the early cohort.
The multivariate analysis revealed that midostaurin retained its independent prognostic value for both OS (HR, 0.55; p = 0.007) and EFS (HR, 0.51; p = 0.001).
Midostaurin and NPM1 mutation
NPM1mut and FLT3mut were present simultaneously in 151 patients. Midostaurin-exposed patients with NPM1mut showed an improved 2-year EFS and 2-year OS compared with midostaurin-naïve patients (Figure 3A).
- Improved OS with midostaurin exposure was maintained in both univariate (HR, 0.50; p = 0.013) and multivariate (HR, 0.40; p = 0.002) analysis.
- Midostaurin exposure was also associated with improved EFS (HR, 0.34; p < 0.001) in the multivariate analysis.
The prognostic value of allelic ratio FLT3-ITD was retained in both cohorts (Figure 3B).
- 2-year OS was higher in patients with FTL3low versus FLT3high in the early cohort.
- In the late cohort, the adverse prognosis of FLT3-ITD was mitigated by an improved 2-year OS in both patients treated for FTL3low versus FLT3high.
- Patients with midostaurin exposure with FLT3low versus FLT3high showed an improved OS (85% vs 67%; p = 0.049) compared with patients who were midostaurin-naïve (67% vs 39%; p = 0.005).
- Patients with NPM1/FLT3low from the late cohort demonstrated a 2-year OS of 81%, and >50% of those patients did not receive allo-HSCT.
- Patients with NPM1/FLT3high who received allo-HSCT in the late cohort showed significantly lower CIR versus the early cohort (30% vs 55%; p = 0.044).
Figure 3. Impact of A midostaurin exposure and B allelic ratio of FLT3-ITD on outcomes*
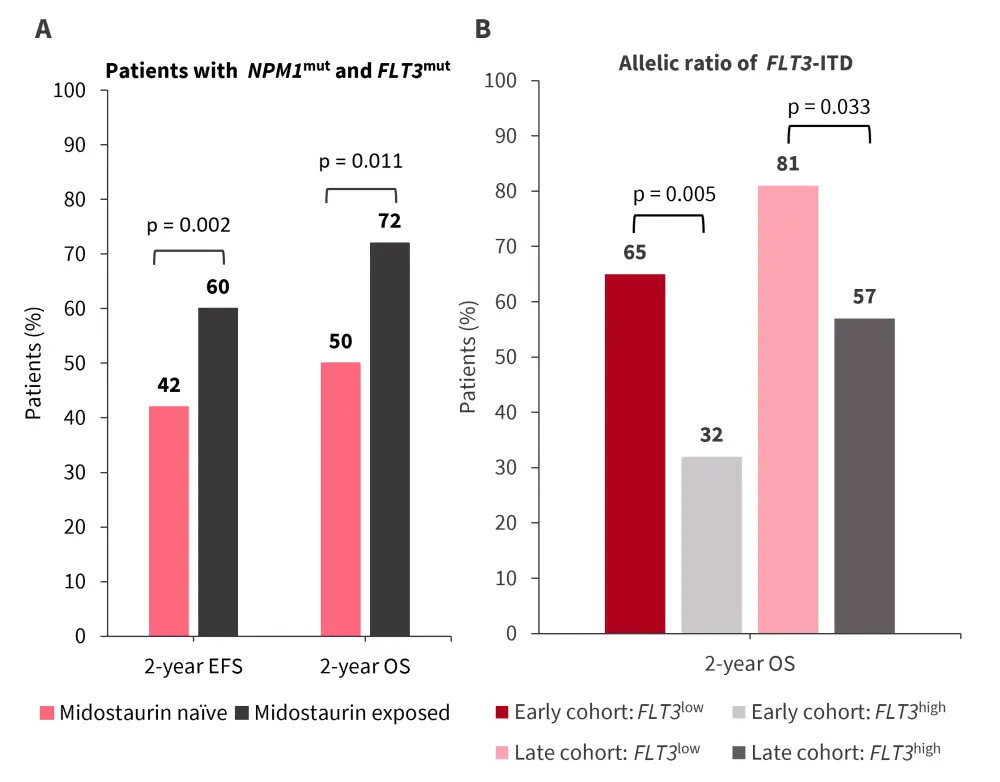
EFS, event-free survival; ITD, internal tandem duplication; OS, overall survival.
*Data from Oñate, et al.1
Midostaurin and wild-type NPM1
- At a median of 10 months, there was no difference in EFS (p = 0.9) or 2-year OS (p = 0.9) in patients with midostaurin exposure versus those who were midostaurin-naïve.
- A higher 2-year CIR (54% vs 33%; p = 0.14) and lower 2-year NRM (8% vs 28%; p = 0.10) was observed in the early versus late cohort.
- Patients with wild-type NPM1 continued to show poor outcomes with a median EFS of 10 months in both cohorts and no difference in 2-year OS (49% vs 48%; p = 0.9) in the early and late cohorts.
Conclusion
This retrospective cohort study confirms the favorable outcome associated with midostaurin in patients with FLT3mut AML eligible for intensive chemotherapy, and particularly in patients aged up to 70 years. Survival outcomes were also significantly improved for patients with NPM1/FLT3 co‑mutation. The findings from the study are consistent with the results of the RATIFY trial, including the impact of midostaurin on molecular subgroups. However, the findings from Oñate et al.1 were limited by the retrospective nature of the study and confounding factors in the late cohort, such as changes in allo-HSCT practice or advances in support measures. Further studies are needed to evaluate midostaurin in the absence of NPM1 mutations and other FLT3 inhibitors to improve outcomes in patients with FLT3mut AML.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



