All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Quizartinib combined with venetoclax and decitabine in FLT3-mutant AML
FLT3 mutations account for ~20–30% of patients with acute myeloid leukemia (AML). Prognosis is poor in older or unfit patients with newly diagnosed FLT3-mutated AML. Quizartinib, a FLT3 inhibitor, has been associated with encouraging response rates and overall survival (OS) in relapsed/refractory (R/R) settings, and it is currently under investigation in different combinations.1
During the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, the results of a phase I/II trial investigating the triple combination of quizartinib, decitabine, and venetoclax for the treatment of FLT3-ITD-mutated AML were presented by Musa Yilmaz, MD Anderson Cancer Center, Houston, US.1
Study design
Primary objective was to identify the recommended phase II dose of quizartinib. Secondary objectives included complete remission (CR), CR with incomplete count recovery (CRi), minimal residual disease (MRD), and OS.
Patient cohorts:
- R/R FLT3-mutant AML or high-risk myelodysplastic syndromes (≥10% blasts)
- Newly diagnosed FLT3-mutant AML unfit for intensive chemotherapy
Study design for the first cycle and subsequent cycles is provided in Figure 1.
Figure 1. Study design*
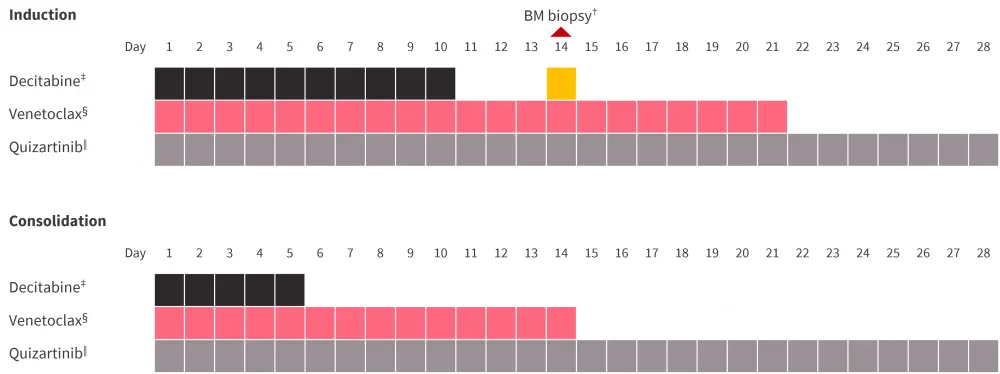
BM, bone marrow; IV, intravenous.
*Adapted from Yilmaz et al.1
†Venetoclax was discontinued on Day 14 in patients with BM blasts ≤5% or hypoblastic BM.
‡20 mg/m2 IV.
§400 mg/day. Based on count recovery durations in Cycle 1, venetoclax duration could be reduced to 14 days or shorter in the subsequent cycles.
‖30 or 40 mg/day.
Results
Twenty-eight patients were included, and Table 1 summarizes the baseline characteristics by patient cohort. All patients had the FLT3-ITD mutation. In the R/R cohort, the median number of prior therapies was 3 (range, 1–5). Almost half of the patients received the combination of hypomethylating agent and venetoclax, 78% and 52% of patients received ≥1 or ≥2 FLT3 inhibitors, respectively.
Table 1. Baseline characteristics*
|
AML, acute myeloid leukemia; ASCT, autologous stem cell transplantation; HMA, hypomethylating agent; R/R, relapsed/refractory; Ven, venetoclax. |
||
|
Characteristic, % |
R/R disease |
Newly diagnosed |
|---|---|---|
|
Median age, years (range) |
57 (23–86) |
69 (65–85) |
|
Male sex |
35 |
20 |
|
AML diagnosis |
||
|
De novo |
78 |
20 |
|
Secondary |
9 |
60 |
|
Therapy-related |
13 |
20 |
|
Prior therapies |
||
|
HMA + Ven |
48 |
— |
|
≥1 FLT3 inhibitor |
78 |
— |
|
≥2 FLT3 inhibitor |
52 |
— |
|
Gilteritinib |
70 |
— |
|
ASCT |
39 |
— |
|
Karyotype |
||
|
Diploid |
39 |
20 |
|
Adverse |
26 |
60 |
|
Additional mutations |
||
|
DNMT3A |
54 |
60 |
|
NPM1 |
45 |
20 |
|
WT1 |
50 |
0 |
|
TET2 |
32 |
0 |
|
RAS/MAPK |
23 |
0 |
|
RUNX1 |
23 |
20 |
|
IDH1/2 |
14 |
20 |
|
TP53 |
5 |
20 |
Nine patients received the combination in phase I (30 mg, n = 7; and 40 mg, n = 2). There was no nonhematologic dose-limiting toxicity (DLT) reported with the 30 mg dose vs two Grade 4 myelosuppression reported with 40 mg. Quizartinib 30 mg/daily was identified as the recommended phase II dose.
Efficacy
Response rate by cohort is depicted in Figure 2. In the newly diagnosed cohort, all patients achieved a response. Other outcomes are summarized in Table 2. There were no deaths within the first 30 days of treatment, one patient in R/R cohort died between Days 30‒60. The 1-year OS was 31%.
Figure 2. Response rate*
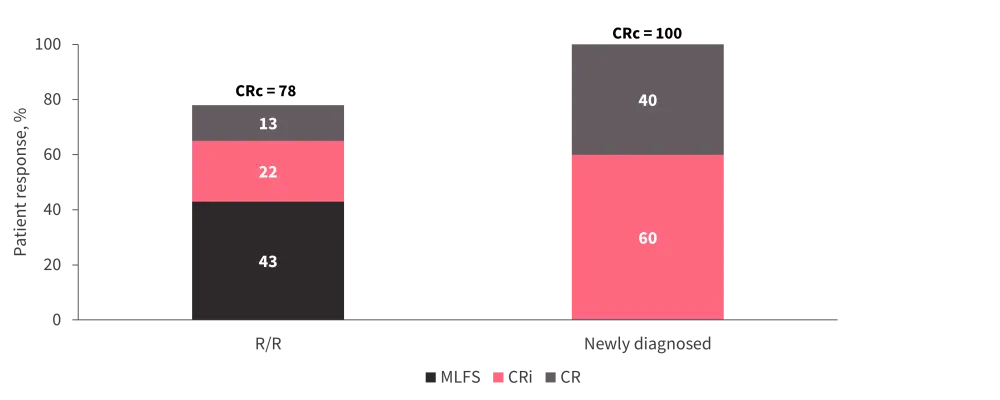
CR, complete remission; CRc, complete response composite; CRi, CR with incomplete count recovery; MLFS, morphologic leukemia-free state; R/R, relapsed/refractory.
*Adapted from Yilmaz et al.1
Table 2. Other efficacy outcomes*
|
ASCT, autologous stem cell transplantation; BM, bone marrow; MRD, minimal residual disease; PCR, polymerase chain reaction; R/R, relapsed/refractory. |
||
|
Outcome, % |
R/R disease |
Newly diagnosed |
|---|---|---|
|
Day 14 BM blasts ≤5% |
52 |
100 |
|
Best MRD, anytime |
||
|
Flow cytometry negativity |
27 |
50 |
|
FLT3 PCR negativity |
38 |
80 |
|
60-day mortality |
5 |
0 |
|
Bridge to ASCT |
34 |
60 |
Subgroup analysis by prior therapies and additional mutations in the R/R cohort showed relatively high composite complete response (CRc) rates (>70%) in patients with prior gilteritinib or hypomethylating agent + venetoclax combination exposure.
Of note, the presence of RAS/MAPK mutations was associated with the lowest CRc (40%) compared with 94% in those without the RAS/MAPK mutations.
- Durable remissions were reported in patients without RAS/MAPK mutations at baseline.
- Among patients who relapsed or were refractory to this regimen, 25% had RAS/MAPK mutations at baseline.
- Among patients who achieved a response but then relapsed, 37% had emerging RAS/MAPK mutations.
- Other emerging mutations were FLT3-F691L and FLT3-ITD negative (25%, each).
Safety
The most common Grade ≥3 nonhematologic treatment-emergent adverse events (TEAEs) included pneumonia (42%), infections (33%), febrile neutropenia (30%), sepsis (9%), leukocytosis, syncope, and hypermagnesemia (6%, each). There were no events of Grade >2 QTcF prolongations.
Due to a prolonged count recovery observed in the first cycles where patients could stay on quizartinib beyond Day 28, the protocol was amended to discontinue quizartinib on Day 28 in patients who were in remission, resulting in improved count recovery (median time to ANC > 500: 40 to 51 days).
Further analyses by cohort
R/R cohort
In a median follow-up duration of 13 months, median OS was 7.6 months.
- Patients with unresponsive disease died due to disease progression (n = 5). Median OS was 5 months.
- Eight patients in remission underwent autologous stem cell transplantation (ASCT); 4 patients were alive in the last follow-up.
- Among those in remission who were unable to undergo ASCT (n = 10), 4 were alive.
- Median OS in patients with RAS/MAPK mutation at baseline was 2.8 months compared to 8.1 months in those without (p = 0.017)
Newly diagnosed cohort
In a median follow-up duration of 16 months, median OS was 14.5 months. Two patients were alive in the last follow-up (both in remission, one underwent ASCT). One patient died while in remission and two patients died due to relapse.
Conclusion
In this trial, the combination of quizartinib (recommended phase II dose of 30 mg/daily), decitabine, and venetoclax was associated with promising response rates and OS. Findings suggest that a delayed count recovery may be managed by dose interruptions. Subgroup analysis revelated that RAS/MAPK mutations were associated with primary and secondary resistance.
Additional content: Click here to read about the results from a phase II trial comparing low-dose cytarabine with or without quizartinib in older patients with AML.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

