All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
The QUAZAR AML-001 study (NCT01757535) has uncovered the significant clinical activity of CC-486 maintenance in patients aged ≥ 55 with acute myeloid leukemia (AML), in first remission following induction chemotherapy and who were not eligible for hematopoietic stem cell transplantation (HSCT).1 The AML Hub is happy to provide a summary of the latest updates from the QUAZAR AML-001 study as presented at this year’s European Hematology Association (EHA) Annual Congress.
CC-486
CC-486 is an orally bioavailable formulation of the hypomethylating agent, azacytidine. Oral administration allows for prolonged therapeutic activity and, in May 2020, the U.S. Food and Drug Administration (FDA) accepted a new drug application and granted priority review to CC-486 for maintenance treatment of adult patients with AML.
QUAZAR AML-001
For the full study design and a summary of the 41.2 month median trial follow-up, click here.
Subgroup analysis
During the EHA annual congress, three subgroup analyses of the QUAZAR study (dose escalation, elderly patients, and patient health-related quality of life [HRQoL]) were presented. The relative study designs can be observed in Figure 1.
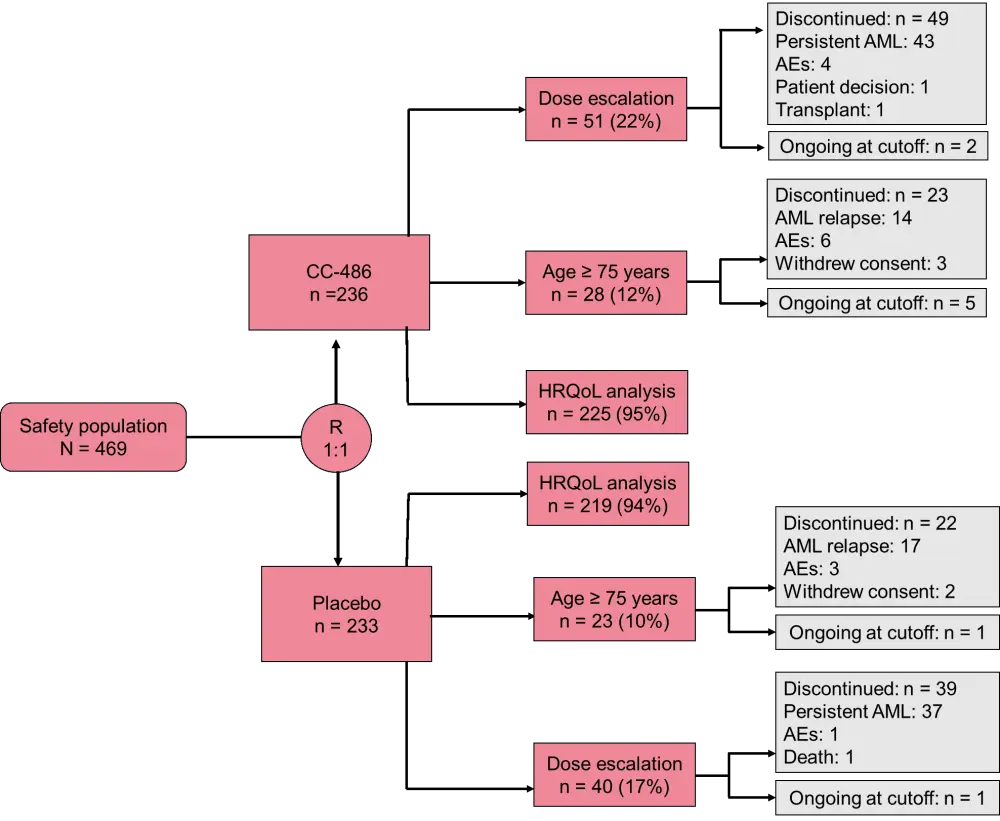
Figure 1. Subgroup analyses of the QUAZAR AML-001 study. AE, adverse event; AML, acute myeloid leukemia; HRQoL, health-related quality of life
Dose escalation1
Initially, patients enrolled in the QUAZAR AML-001 study received the study drug for 14 days per 28-day cycle. However, an alternative, escalated 21-day dosing schedule has been suggested for patients experiencing early AML relapse with 5 – 15% blasts in peripheral blood or bone marrow. During the EHA 2020, Hartmut Döhner, Ulm University Hospital, Ulm, DE, presented the results from the dose-escalation cohort of the QUAZAR AML-001 study.
Results
- Study characteristics are presented in Table 1
- Seventy-eight patients had bone marrow with ≥ 5% blasts on or before the first day of the dosing cycle
- Fourteen patients achieved a second complete remission during dose escalation
Table 1. QUAZAR AML-001 dose escalation study characteristics1
|
Dose escalation (n = 91) |
CC-486 (n = 51) |
Placebo (n = 40) |
|---|---|---|
|
Median time to dose escalation, months (range)
|
9.2 (1.0–52.7) |
6.0 (0.5–19.3) |
|
Median number of escalated dosing cycles |
2 |
2 |
|
Patients receiving > 3 cycles of escalated dosing, % |
43 |
18 |
Efficacy
- Patient outcomes observed in the dose-escalation cohort are presented in Figure 2
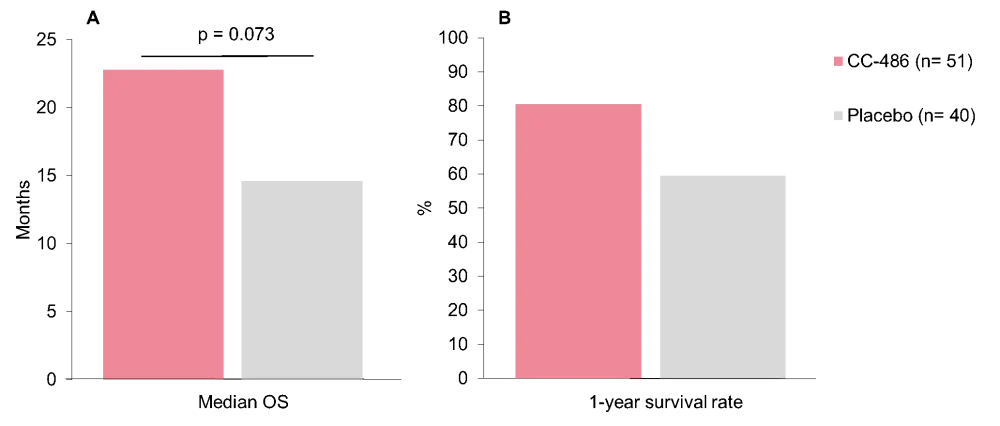
Figure 2. A Median OS and B 1-year survival rates among patients that received an escalated, 21-day dosing schedule of CC-486 vs placebo in the QUAZAR AML-001 study. OS, overall survival
Safety
- The frequency of Grade 3–4 adverse events (AEs) were comparable between patients in the CC-486 and placebo arms with the exception of febrile neutropenia and neutropenia which were observed more frequently in patients receiving CC-486 (Table 2)
- Dose escalation of CC-486 did not have a negative impact on overall HRQoL or on patient-reported measures of fatigue
Table 2. Grade 3–4 AEs observed in patients receiving an escalated, 21-day dosing schedule of CC-486 vs placebo1
|
AE, adverse event |
||
|
AE, % |
CC-486 (n = 51) |
Placebo (n = 40) |
|---|---|---|
|
≥ 1 Grade 3–4 AE |
31 |
35 |
|
Febrile neutropenia |
24 |
3 |
|
Neutropenia |
22 |
13 |
|
Thrombocytopenia |
18 |
30 |
|
Anemia |
16 |
18 |
|
Fatigue |
6 |
0 |
|
Constipation |
6 |
0 |
|
Pneumonia |
4 |
5 |
|
Sepsis |
2 |
5 |
Study conclusions
In patients with AML experiencing early relapse with 5 – 15% blasts, the 21-day dose escalation demonstrated clinical efficacy and should be considered for routine treatment of these patients. No additional safety concerns were associated with the escalated dosing schedule and, although rates of febrile neutropenia and neutropenia were elevated in the CC-486 arm, hematological AEs were consistent with AML relapse.
Elderly patients2
Farhad Ravandi, MD Anderson Cancer Center, Houston, US, presented results from a subgroup analysis evaluating the safety and tolerability of CC-486 in patients aged ≥ 75 years at the time of enrollment onto the QUAZAR AML-001 study.
Results
- Baseline characteristics of patients aged ≥ 75 years at enrollment are presented in Table 3
- Median treatment duration was longer in patients receiving CC-486 vs placebo
Table 3. Baseline characteristics of patients aged ≥ 75 years at the time of enrollment2
|
AML, acute myeloid leukemia; CR, complete remission, CRi, complete remission with incomplete hematologic response; ECOG PS, Eastern Cooperative Oncology Group (ECOG) Performance Status; MRD, measurable residual disease; WHO, World Health Organization |
||
|
Characteristic |
CC-486 (n = 28) |
Placebo (n = 23) |
|---|---|---|
|
Sex, % female |
50 |
52 |
|
Cytogenic risk at diagnosis, % Intermediate Poor |
82 18 |
87 13 |
|
Response following induction, % CR CRi |
75 25 |
83 17 |
|
Received consolidation, % |
57 |
70 |
|
ECOG PS score at screening, % 0 1 2–3 |
50 39 11 |
65 30 4 |
|
MRD+ at consolidation, % |
36 |
52 |
|
De novo AML, % |
86 |
100 |
|
WHO AML classification Not otherwise specified Myelodysplasia-related changes Recurrent genetic abnormalities Therapy-related |
61 29 11 0 |
48 13 39 0 |
Efficacy
- In patients aged ≥ 75 years, maintenance with CC-486 improved overall survival and remission-free survival vs placebo (Figure 3)
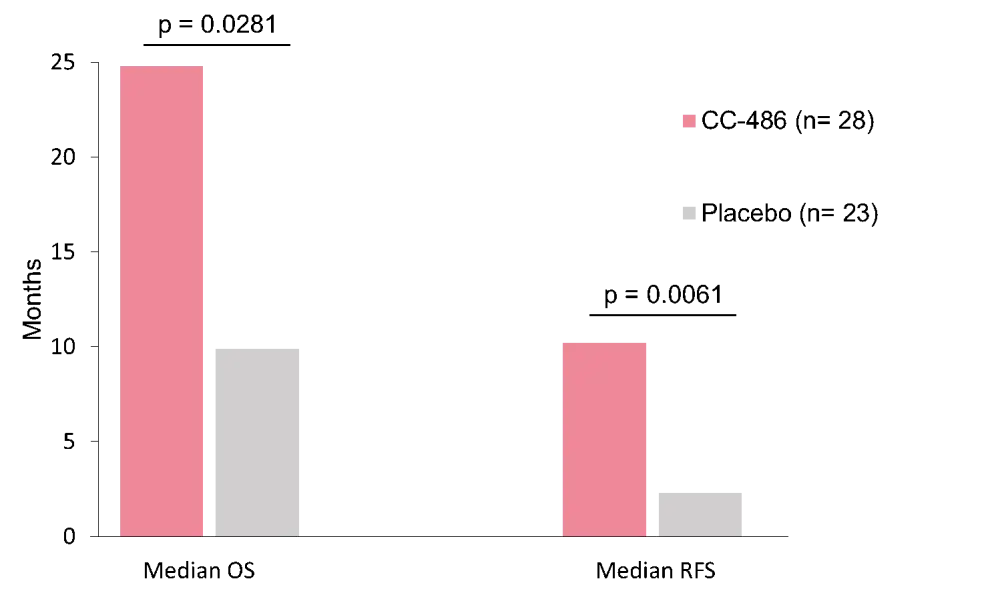
Figure 3. Survival outcomes among patients aged ≥ 75 years at the time of enrollment. OS, overall survival; RFS, relapse-free survival
Safety
- In patients aged ≥ 75 years:
- Gastro-intestinal AEs were the most common, and were observed more frequently in the CC-486 vs placebo arm
- diarrhea was the most common AE resulting in treatment discontinuation
- As in the dose escalation study
- Grade 3–4 neutropenia was observed more frequently in the CC-486 vs placebo arm
- Grade 3–4 thrombocytopenia was observed more frequently in the placebo vs CC-486 arm
- Gastro-intestinal AEs were the most common, and were observed more frequently in the CC-486 vs placebo arm
Table 4. Grade 3–4 AEs reported in ≥ 10% of patients aged ≥ 75 years at the time of enrollment2
|
AE, adverse event |
||
|
AE, % |
CC-486 (n = 51) |
Placebo (n = 40) |
|---|---|---|
|
Febrile neutropenia |
14 |
0 |
|
Neutropenia |
43 |
13 |
|
Thrombocytopenia |
11 |
30 |
|
Anemia |
14 |
13 |
|
Diarrhea |
11 |
4 |
Study conclusions
CC-468 demonstrated favorable clinical efficacy and tolerability in patients in first complete remission (CR) aged ≥ 75 years at the time of study enrollment. No additionally safety concerns were highlighted in this patient subgroup and treatment with CC-486 may overcome the increased risk of relapse observed in elderly patients with AML.
Long-term follow up — QUAZAR AML-001 and HRQoL3
As one of the major debilitating symptoms of AML, fatigue negatively impacts patient HRQoL. An optimal maintenance therapy should not exacerbate fatigue. At this year’s EHA meeting, Gail Roboz discussed the HRQoL of patients enrolled on the QUAZAR AML-001 study.
Study design
- Primary HRQoL endpoint: Mean changes from baseline in the HRQoL score (CC-486 vs placebo)
- Secondary HRQoL endpoints:
- Rates of clinically significant improvement or deterioration from baseline
- Time to definitive clinically significant sustained deterioration
- The functional assessment of chronic illness therapy (FACIT)-Fatigue and EQ-5D-3L were used to assess fatigue and overall HRQoL, respectively
Results
- Low levels of fatigue and generally good HRQoL were observed across the CC-486 and placebo arms (Table 5)
- Low levels of clinically meaningful differences were observed across the CC-486 and placebo arms
- There were no differences in overall change of FACIT-Fatigue or EQ-5D-3L scores between study arms
- Ongoing treatment with CC-486 was well tolerated, as demonstrated by the median number of treatment cycles (Table 5)
Table 5. Baseline characteristics of patients eligible for HRQoL analysis3
|
CRi, complete remission with incomplete hematologic response; ECOG PS, Eastern Cooperative Oncology Group (ECOG) Performance Status; FACIT, functional assessment of chronic illness therapy; HRQoL, health-related quality of life; HUI, health utility index; MID, minimally important difference; VAS, visual analogue scale |
||
|
Characteristic |
CC-486 (n = 225) |
Placebo (n = 219) |
|---|---|---|
|
Median age, years (range) |
68 (55–86) |
68 (55–82) |
|
Sex, % female |
51 |
46 |
|
Cytogenic risk at diagnosis, % Intermediate Poor |
87 13 |
88 12 |
|
Response following induction, % CR CRi |
78 22 |
84 16 |
|
Received consolidation, %
|
79 |
82 |
|
ECOG PS score at screening, % 0 ≥ 1 |
50 50 |
50 50 |
|
Median number of treatment cycles, n |
12 |
7 |
|
Mean FACIT-Fatigue score |
40.8 |
40.7 |
|
Mean EQ-5D-3L HUI score |
0.80 |
0.79 |
|
Mean EQ-5D-3L VAS score |
74.6 |
75.4 |
Study conclusions
Good overall HRQoL and low levels of fatigue were demonstrated by patients in CR or CR with incomplete hematologic response, and this HRQoL was sustained over the entire course of maintenance treatment with CC-486. Treatment with CC-486 did not worsen patient quality of life and CC-486 maintenance significantly improved overall survival and remission-free survival.
Overall conclusions
Data presented at this year’s EHA annual congress consolidate the potential of CC-486 as a maintenance strategy for the treatment of patients aged ≥ 55 with AML in first remission. CC-486 has demonstrated favorable tolerability in elderly patients, does not significantly impact patient HRQoL and improves patient survival outcomes regardless of the consolidation therapy received, and across subgroups. Furthermore, accelerated 21-day dosing schedule appears well-tolerated and effective, providing an alternative treatment approach to patients experiencing early relapse.
Expert Opinion
 Andrew Wei
Andrew WeiReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

