All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Patients with pretransplant MRD may benefit from conditioning intensification
Featured:
At the 46th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), the AML Hub will hold a satellite symposium on how measurable residual disease (MRD) status impacts the outcome of stem cell transplantation (SCT) in patients with acute myeloid leukemia (AML). Here, we present an article demonstrating that conditioning intensification for patients with AML with pretransplant MRD can reduce relapse and improve survival.
Christopher S. Hourigan and colleagues retrieved the data from the randomized phase III MAvRIC trial and analyzed patient MRD status before assignment to either myeloablative conditioning (MAC) or reduced-intensity conditioning (RIC). Their results were selected as late-breaking in the 2019 presidential plenary of the 24th European Hematology Association (EHA) meeting and were recently published in the Journal of Clinical Oncology.1
Study design1
This study analyzed a total of 190 pretransplant frozen whole blood samples from patients with AML enrolled in the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901 trial (MAvRIC, NCT01339910). All patients were in morphologic complete response at the time of sample collection and were eligible for undergoing an allogeneic SCT (allo-SCT).
All samples were analyzed by error-corrected ultra-deep next-generation sequencing (NGS) for the presence/absence of mutations in 13 genes, which are frequent aberrations in AML: ASXL1, DNMT3A, FLT3, IDH1, IDH2, JAK2, KIT, NPM1, NRAS, RUNX1, SF3B1, TET2, and TP53.
Results1
Patient baseline characteristics were balanced between treatment arms (Table 1), even in the proportion of patients without detectable mutations before transplantation (32% vs 37% of patients treated with MAC and RIC, respectively). Interestingly, patients with NGS positivity were significantly older than those with negative NGS (57 vs 50 median years, respectively; p < 0.001), although this did not impact the study results.
Mutations in DNMT3A, TET2, and ASXL1 (DTA mutations) were detected in 45% of patients and frequently co-occurred in the same sample. The presence of mutations in DTA genes is associated with aging and does not seem to have a prognostic impact on the clinical outcome of patients with AML. Therefore, the analysis was also performed with the alternative definition of NGS-positive status: considering mutations in all the listed genes except the DTA group. This resulted in NGS-positive classification in 41% of all patients: 42% and 40%, treated with MAC and RIC, respectively.
Table 1. Patient clinical characteristics1
|
Bu, busulfan; Cy, cyclophosphamide; Flu, fludarabine; HCT-CI, hematopoietic cell transplant comorbidity index; MAC, myeloablative conditioning; Mel, melphalan; NA, not applicable; RIC, reduced-intensity conditioning; TBI, total body irradiation |
||
|
Characteristic |
MAC n = 95 patients |
RIC n = 95 patients |
|---|---|---|
|
Median age, years |
54.9 |
54.7 |
|
Median disease duration, months |
4.9 |
4.4 |
|
Cytogenetics, % |
||
|
Favorable |
5.3 |
11.6 |
|
Intermediate |
62.1 |
54.7 |
|
Poor |
27.4 |
29.5 |
|
Not tested or unknown |
5.3 |
4.2 |
|
HCT-CI, % |
||
|
0 |
34.7 |
34.7 |
|
1–2 |
34.7 |
31.6 |
|
> 2 |
30.5 |
33.7 |
|
Conditioning regimen, % |
||
|
Flu/Bu4 |
56.8 |
NA |
|
Bu/Cy |
39.0 |
NA |
|
Cy/TBI |
4.2 |
NA |
|
Flu/Mel |
NA |
17.9 |
|
Flu/Bu2 |
NA |
82.1 |
Patients who received MAC experienced higher transplant-related mortality than those who received RIC, although the latter relapsed earlier (Table 2). The median follow-up in surviving patients was > 49 months; 77% of the relapses occurred in the first year after allo-SCT.
When considering the detection of mutations pretransplant, i.e., NGS positive or negative,
- patients who were NGS positive relapsed more frequently, with highest rates reported in the RIC arm
- overall survival was similar between conditioning arms for patients who were NGS negative, but those with detectable mutations pretransplant experienced a significantly higher relapse and inferior survival when treated with RIC than with MAC
- These results were further confirmed when considering a refined definition of NGS positivity (only non-DTA mutations) (Figure 1)
Figure 1. Overall survival reported in patients negative by NGS and with DTA mutations versus patients NGS positive with non-DTA mutations, treated with MAC or RIC. Original figure provided by the first author, Christopher S. Hourigan.1
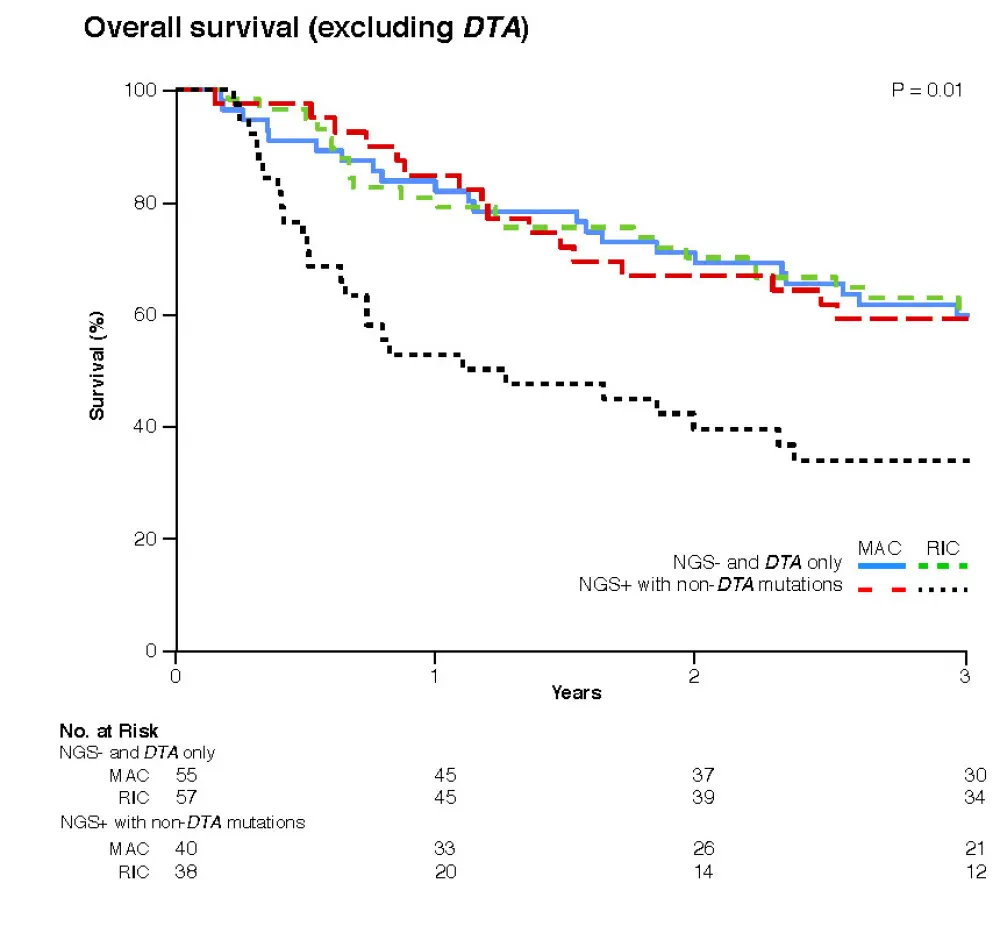
DTA, grouped genes DNMT3A, TET2, and ASXL1; MAC, myeloablative conditioning; NGS, next-generation sequencing; RIC, reduced-intensity conditioning
Since the diagnostic sample was not available for comparison, the investigators could not assume that the detected mutations pretransplant were from the original leukemic clone. Nevertheless, they were able to describe the different prognostic impact on relapse associated with detected mutations in each arm. Excluding patients who died due to transplant-related complications,
- for patients with detectable IDH1, IDH2, SF3B1, and/or non-ITD FLT3 mutations, none of those treated with MAC relapsed, while all those who received RIC experienced a relapse
- NPM1 and FLT3-ITD mutations were associated with higher relapse rates, irrespective of the conditioning regimen
Table 2. Clinical endpoints in patients treated with MAC or RIC, according to their NGS status1
|
DTA, grouped genes DNMT3A, TET2, and ASXL1; MAC, myeloablative conditioning; NGS, next-generation sequencing; OS, overall survival; RIC, reduced-intensity conditioning; TRM, transplant-related mortality *NGS positive if mutations are detected in any of the following genes: ASXL1, DNMT3A, FLT3, IDH1, IDH2, JAK2, KIT, NPM1, NRAS, RUNX1, SF3B1, TET2, and TP53. |
|||
|
|
MAC |
RIC |
p value |
|---|---|---|---|
|
Median time to relapse, months |
8.1 |
3.3 |
|
|
1-year relapse rate, % |
15 |
47 |
< 0.001 |
|
NGS positive* |
14 |
58 |
< 0.001 |
|
3-year relapse rate in NGS positive for non-DTA mutations, % |
15 |
72 |
< 0.001 |
|
3-year TRM, % |
27 |
9 |
0.001 |
|
3-year OS, % |
|
|
|
|
NGS negative |
56 |
63 |
0.96 |
|
NGS positive* |
61 |
43 |
0.02 |
|
NGS positive for non-DTA mutations |
59 |
34 |
0.01 |
Conclusion
Patients with genomic detectable disease pretransplant treated with a RIC regimen experienced an increased risk of relapse and inferior overall survival compared with patients treated with MAC. The question of whether a MAC regimen should be prioritized when feasible for these patients needs to be addressed. Furthermore, patients with MRD not eligible for MAC may benefit from additional chemotherapy or novel agents pretransplant and posttransplant, to reduce their risk of early relapse.1
On the other hand, patients with negative NGS before allo-SCT had similar relapse and survival outcomes despite the intensity of the conditioning received.1 Ideally, MRD and genomic analyses will be incorporated in all future trials, to study the MRD dynamics and effectively identify those patients who could benefit the most from a RIC approach and be spared of the additional toxicity.2
Future studies will be needed to overcome some limitations of this analysis, such as the availability of bone marrow samples, the possibility of correlating the NGS results with the diagnostic samples, and comparing NGS with other techniques used for MRD assessment.
During the AML Hub Satellite Symposium, five international experts—Gert Ossenkoppele, Jacqueline Cloos, Christian Thiede, Adriano Venditti, and Charles Craddock—will discuss the latest developments and remaining challenges of MRD assessments in the context of SCT. Join us on August 30, 2020 (8:30 am CEST)!
Expert Opinion
“This study provides good evidence that detection of measurable residual disease in AML is not just a prognostic marker for an inevitable poor outcome,” said Dr. Hourigan DM DPhil FRCP FACP, Chief of the Laboratory of Myeloid Malignancies at the National Heart, Lung, and Blood Institute, part of the National Institutes of Health, and lead author on this study. “The findings show that we, as doctors, can intervene on this state and improve clinical outcomes by personalizing our treatment in this high-risk blood cancer. The challenge now is to find the optimal therapy for AML MRD in each individual.”
 Christopher Hourigan
Christopher HouriganReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

