All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Management of adverse events during oral azacitidine maintenance: a trial update from QUAZAR AML-001
We have extensively covered results from the phase III QUAZAR AML-001 trial, highlighting improved overall survival and relapse-free survival with oral azacitidine maintenance versus placebo in elderly patients in first complete remission. Less detailed is the impact of oral azacitidine maintenance on health-related quality of life in these patients. In addition to gastrointestinal (GI) symptoms, myelosuppressive effects were commonly reported in this trial.1 It is important to analyze the management of adverse events (AEs), and fully detail their onset.
In our latest trial update, we summarize a safety analysis of QUAZAR AML-001 that investigated commonly observed GI and hematologic events, as well as practical recommendations to manage such symptoms. This analysis was recently published by Ravandi et al.1 in the Journal of Hematology & Oncology.
Methods
For a summary of the trial design, including eligibility criteria, click here.
Safety was assessed in all patients who received ≥1 dose of the study drug.
GI adverse events
We recently reported GI AEs, which were mostly low grade, in our editorial theme. We also summarized early intervention of GI symptoms with concomitant medication or azacitidine dose modifications.
Hematologic AEs
- A total of 66% of patients receiving oral azacitidine reported hematologic AEs, which were most commonly neutropenia (44%), thrombocytopenia (33%) and anemia (20%).
- The rate of AEs produced a downward trend throughout the first six azacitidine treatment cycles.
- During Cycles 1–2, 3–4, and 5–6, rates of neutropenia were 22%, 20%, and 18%, respectively.
- The rates of thrombocytopenia were 16%, 9%, and 6%.
- The rates of anemia were 6%, 4%, and 4%.
- Grade 3–4 neutropenia, thrombocytopenia, and anemia were reported in 41%, 22%, and 14% of patients, respectively.
- Serious febrile neutropenia was reported in 7% of patients, compared with 4% in patients receiving placebo.
- Notably, the authors described a possible overlap of cytopenia due to oral azacitidine and those who relapse.
- A total of 65% of patients relapsed, and it is unclear how many of the hematologic AEs were related to this.
Management of hematologic AEs
- Modifications to azacitidine treatment according to hematologic AEs are summarized in Figure 1.
Figure 1. Azacitidine dose modifications*
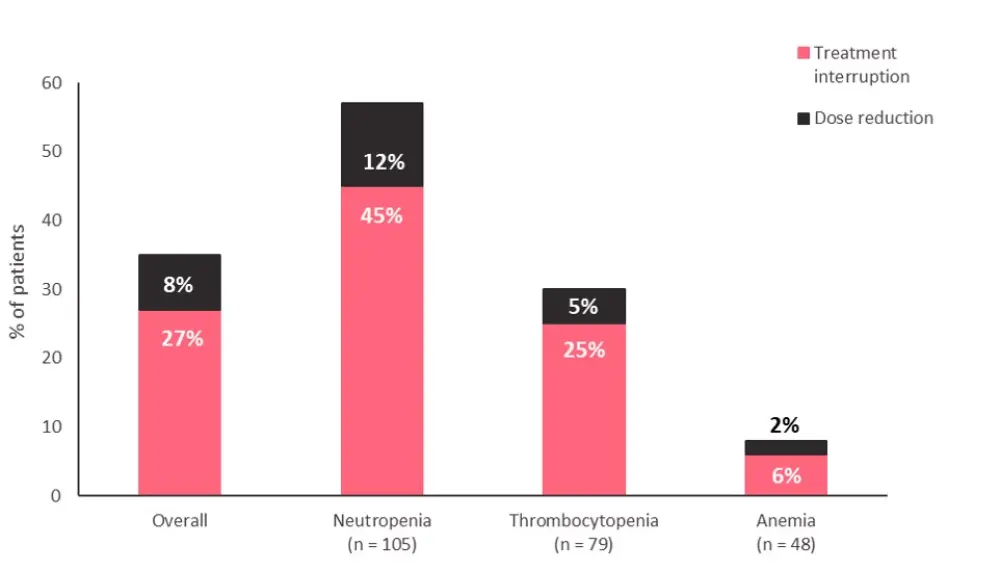
*Data from Ravandi, et al.1
- Hematologic AEs in the QUAZAR trial were primarily managed with azacitidine dose modifications, mostly pertaining to treatment interruption, as highlighted in Figure 1.
- Only three patients discontinued azacitidine treatment due to hematologic AEs.
- The guidance of dose modifications for azacitidine maintenance are summarized in Figure 2.
- In the QUAZAR AML-001 trial, the authors noted that patients with febrile neutropenia continued to receive azacitidine or placebo alongside antibiotic, antifungal, or antimicrobial agents, up to 3 days before interruption and no earlier than 3 days after recovery.
Figure 2. Dose modification of azacitidine according to hematologic AEs*
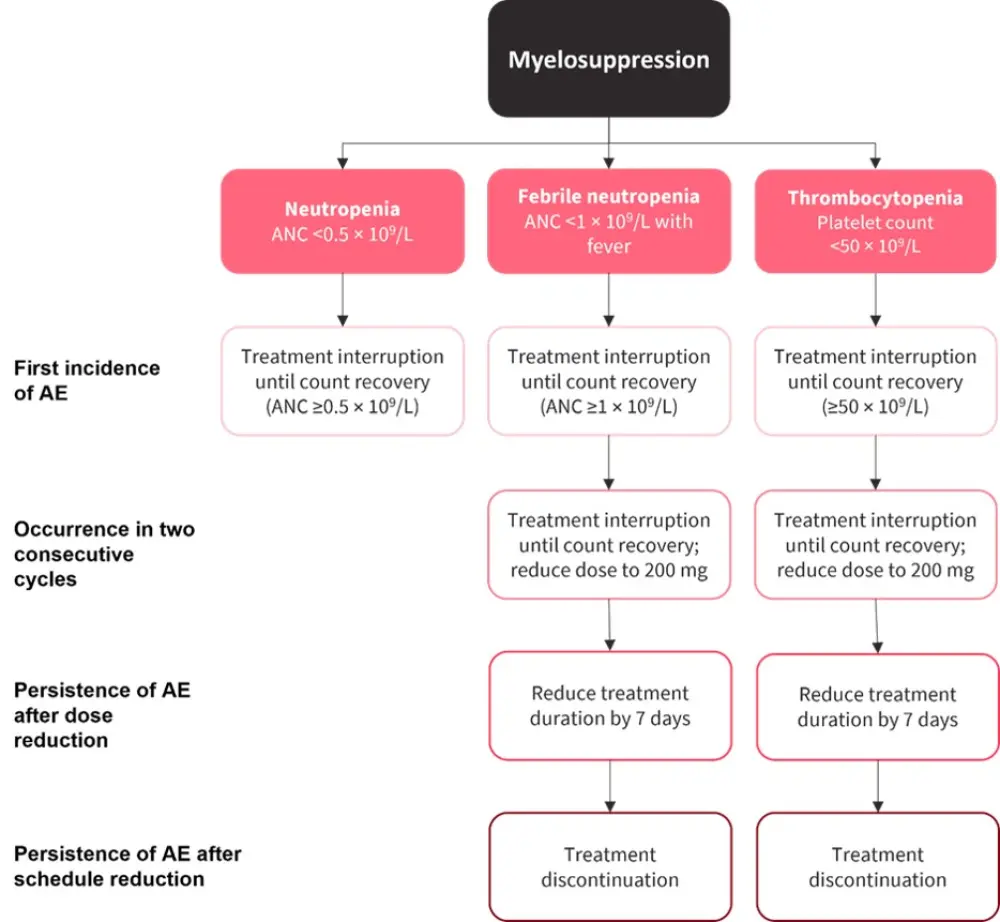
AE, adverse event; ANC, absolute neutrophil count.
*Adapted from Ravandi, et al.1
- In terms of prophylactic treatment, the following were given to patients for hematologic AEs:
- Neutropenia: Myeloid growth factors, including granulocyte-colony stimulating factor (G-CSF)/filgrastim were used in 21% of patients.
- Febrile neutropenia: G-CSF/filgrastim was not given as the overall risk of febrile neutropenia was 12%, lower than the expert guidelines threshold of ≥20%.
- Thrombocytopenia: Platelet transfusions were given to 19% of patients receiving azacitidine, while one patient received prothrombin.
- Anemia: Red blood cell transfusions were given to 23% of patients, and one patient was given epoetin alpha, an erythropoietin stimulating factor.
Infections
- A total of 62% of patients were reported to have infections while receiving azacitidine, and 12% were related to treatment.
- The most common infections were upper respiratory tract infections (13%) influenza (8%), nasopharyngitis (7%), urinary tract infections (7%), and pneumonia (6%).
- Grade 3–4 pneumonia was reported in 3% of patients.
- Serious infections in >1 patient were pneumonia (4%), cellulitis (2%), sepsis (2%), and influenza (1%).
- A total of three patients died due to infections while receiving oral azacitidine; however, these where all deemed to be unrelated to treatment, instead occurring due to AML relapse.
Management of infections
- A total of 13% of patients had treatment interrupted due to infections, while 1% had their dose reduced, and treatment was discontinued for four patients.
- Treatment discontinuation was due to sepsis, pneumonia, rectal abscess, or lung abscess.
- Sepsis was considered by the investigator to be related to oral azacitidine.
- In accordance with ASCO and Infectious Diseases Society of America (IDSA) guidelines, concomitant antifungal and antibacterial treatment was given as a result of increased risk of neutropenia (<10 × 109/L for 7 days).
- The most common concomitant antibiotics, anti-infectives, and antifungal treatments during the trial were ciprofoxacin (22%), levofoxacin (22%), and acyclovir (20%).
- Also highlighted in this analysis was the potential to use G-CSF to reduce the incidence of infection-related mortality.
Conclusion
Overall, this study demonstrated a manageable safety profile for oral azacitidine maintenance, with a low number of patients discontinuing treatment. Similar to GI effects, hematologic AEs were controlled mostly through dose reductions and treatment interruptions, in adherence with expert guidelines. Implementation of these interventions will help improve treatment adherence and survival outcomes. Regarding the overlap of cytopenia in cases of relapse and azacitidine treatment, investigators noted that persistent neutropenia and cytopenia following dose modification and schedule changes may signal oncoming relapse.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

