All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
CC-486 maintenance prolongs survival in older patients with AML in first remission
Visual abstract
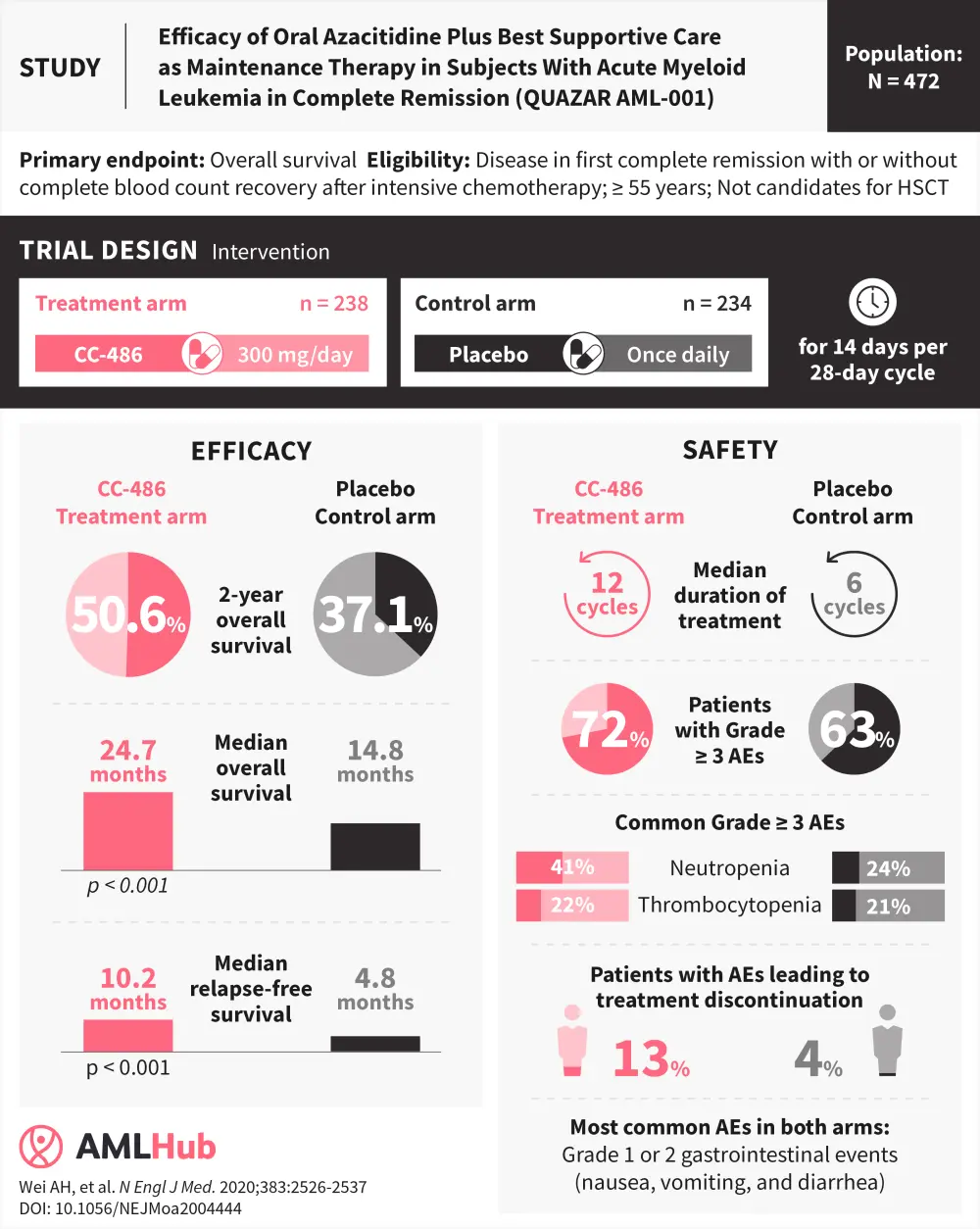
Despite reasonable initial responses with standard induction chemotherapy in elderly patients with acute myeloid leukemia (AML), approximately 80–90% of patients eventually relapse. In patients with AML, the duration of first remission is positively associated with favorable survival, and so it becomes crucial to prolong remission and prevent early relapse using post-remission therapy. Hematopoietic stem cell transplantation (HSCT) is often used as a potentially curative option following induction chemotherapy, however, older patients with AML are not always eligible for such intensive approaches.
Effective AML maintenance therapies are required when HSCT is not feasible and should reduce the risk of relapse while maintaining patient health-related quality of life. The oral formulation of azacitidine, CC-486, was approved as a maintenance therapy by the U.S Food and Drug Administration (FDA) in September 2020. The approval was based on the results of the phase III QUAZAR AML-001 trial (NCT01757535), evaluating maintenance with CC-486 vs placebo in patients aged ≥ 55 years with AML in first remission. Topline results showed that maintenance with CC-486 resulted in significantly improved overall survival (OS; the primary endpoint) and relapse-free survival (RFS; a secondary endpoint) vs placebo in the elderly AML setting.
Results from the follow-up of the QUAZAR AML-001 trial were presented at the 61st American Society of Hematology (ASH) Meeting and Exposition in 2019, and have since been published by Andrew Wei and colleagues in The New England Journal of Medicine.1 Read the AML Hub summary here, which highlights the clinically significant improvements in OS and RFS for patients in first remission receiving CC-486 as a maintenance therapy. The AML Hub is happy to provide a visual abstract of the QUAZAR AML-001 study.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

