All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Long-term follow-up post hoc analysis of sorafenib maintenance therapy after allo-HSCT in patients with FLT3-mutated AML
Do you know... Which of the following statements would you expect of patients with AML with FLT3 internal tandem duplications compared to those with FLT3-wild type AML?
Approximately 25% of adults with acute myeloid leukemia (AML) have FLT3 internal tandem duplication (FLT3-ITD) mutations, which are associated with poor survival outcomes.1 Allogeneic hematopoietic stem cell transplantation (allo-HSCT) can improve survival in these patients. However, FLT3-ITD mutations are associated with increased risk of relapse. Sorafenib is a multi-kinase FLT3-ITD inhibitor that has demonstrated improved relapse rates when used as a maintenance therapy in patients with FLT3-ITD AML after allo-HSCT. The SORMAIN trial, previously reported by the AML Hub, demonstrated a 2-year overall survival (OS) of 90.5% with sorafenib maintenance versus 66.2% with placebo (p = 0.007). However, there are limited data on long-term follow-up of sorafenib maintenance.
The 5-year follow-up results of a phase III trial investigating the use of sorafenib maintenance post-transplantation in patients with FLT3-mutated AML were recently published by Xuan et al. in The Lancet.1 We are pleased to present a summary of this post hoc long-term analysis here.
Study design
This post hoc analysis was of a multicenter, open-label, randomized trial (NCT02474290) in China. Patients with FLT3-ITD AML undergoing first allo-HSCT were eligible. The study design is shown in Figure 1.
For this long-term analysis, the 5-year endpoints were as follows:
- OS – time from transplantation until death from any cause
- Cumulative incidence of relapse
- Non-relapse mortality (NRM) – death from any cause not subsequent to relapse
- Leukemia-free survival (LFS) – time from transplantation until relapse or death from any cause
- Graft-versus-host-disease (GvHD)-free, relapse-free survival (GRFS) – time from transplantation until Grade 2–4 acute GvHD, chronic GvHD (cGvHD) requiring systemic immunosuppressive therapy, relapse, or death from any cause
- Cumulative incidence of cGvHD
- Late effects – evaluated in patients who were leukemia free for at least 6 months after transplantation
Figure 1. Study design*

aGvHD, acute graft-versus-host disease; allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia;
ECOG PS, Eastern Cooperative Oncology Group performance status.
*Adapted from Xuan et al.1
†Includes complete remission, complete remission with incomplete platelet recovery, and complete remission with incomplete hematological recovery.
‡Defined as an absolute neutrophil count of 1.0 x 109 cells/L or higher and non-transfused platelets of 30 × 10⁹ cells/L or higher for at least 3 consecutive days.
§Intravenous busulfan (0·8 mg/kg four times per day on Days 4–7 before transplantation), intravenous cyclophosphamide (60 mg/kg once daily on Days 2–3 before transplantation), and oral semustine (250 mg/m² on Day 3 before transplantation).
Results
A total of 202 patients were included, 100 in sorafenib maintenance and 102 patients in the control arm, and median follow-up was 60.4 months. Sorafenib maintenance showed improved OS, LFS, and GRFS versus control arm (Figure 2). The median LFS was not reached in the sorafenib maintenance arm versus 35.8 months in the control arm.
Figure 2. Five-year survival outcomes*

GFRS, graft-versus-host-disease relapse-free survival; LFS, leukemia-free survival; NRM, non-relapse mortality; OS, overall survival.
*Adapted from Xuan et al.1
Relapse
- The median time to relapse after transplantation was 11.9 versus 6.5 months in the sorafenib and control arms, respectively (p = 0.25).
- Relapse occurred in 15% and 36% of patients in the sorafenib maintenance and control arms, respectively.
- Of these patients, relapse after transplantation occurred at:
- 12–24 months in 53% (8/15) of patients in the sorafenib maintenance arm
- 6 months in 46% (17/37) of patients in control arm.
- Of the 15 patients who relapsed in the sorafenib group, three patients relapsed within 6 months of stopping treatment. The median time to relapse was 5.6 months after stopping sorafenib.
- These patients had measurable residual disease (MRD)-positive status at transplantation and were MRD negative at Day 180 after transplantation.
- At 18 months after stopping sorafenib, 73% (11/15) of patients in the sorafenib maintenance arm relapsed.
- Of these 64% (7/11) of patients were MRD-positive at transplantation.
- The remaining patient relapsed at 25.3 months after stopping sorafenib.
Risk factors of survival and relapse
- A total of 73 patients died: 28 were on sorafenib maintenance and 45 were in the control arm (Figure 3).
- Multivariable analysis showed that sorafenib maintenance after transplantation (hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.32–0.85; p = 0.0087) and cGvHD (HR, 0.50; 95% CI, 0.31–0.80; p = 0.0044) were protective factors associated with OS.
- The only risk factor associated with OS was the MRD status at transplantation (positive vs negative) (HR, 1.76; 95% CI, 0.97–3.21; p = 0.064).
Figure 3. Causes of death*

GvHD, graft-versus-host disease.
*Adapted from Xuan et al.1
†Infection-related and not related to sorafenib.
Genetic subgroup analysis
A total of 71% of all patients harbored at least one co-occurring mutation. The most common co-occurring mutations (in ≥5% of patients) included NPM1 (27%), DNMT3A (15%), TET2 (11%), CEBPA (11%), IDH2 (7%), and RUNX1 (6%). Triple mutations (NPM1, DNMT3A, and FLT3-ITD) occurred in 10% of patients. Of the 15% and 36% of patients who relapsed in the sorafenib maintenance and control arms, 40% and 49% had FLT3-mutated AML, respectively. Figure 4 shows OS, cumulative incidence of relapse, and LFS at 5 years in the genetic subgroups in sorafenib maintenance and control arms.
Figure 4. Genetic subgroup analysis of A overall survival, B cumulative incidence of relapse, and C leukemia-free release*
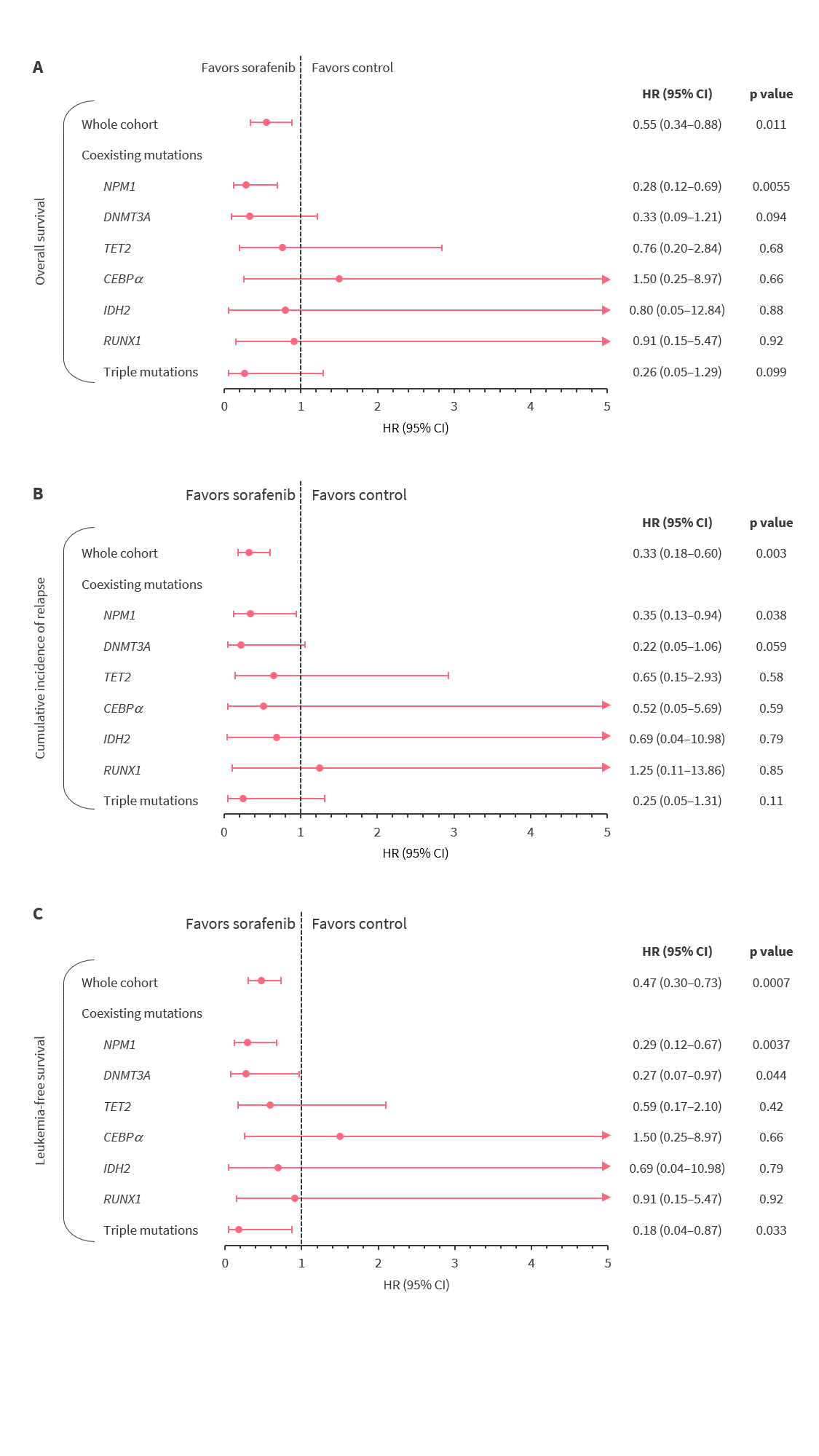
CI, confidence interval; HR, hazard ratio.
*Adapted from Xuan et al.1
Chronic GvHD and late effects
A total of 53% of patients developed cGvHD: 54% in the sorafenib maintenance and 51% in the control arm (Figure 5).
Figure 5. Chronic GvHD – sorafenib maintenance versus control*

cGvHD, chronic graft-versus-host disease.
*Adapted from Xuan et al.1
Late effects were analyzed in 174 patients who were leukemia-free for ≥6 months post-transplant, 55% of these were in sorafenib maintenance and 45% in the control arm (Figure 6).
Figure 6. Late effects – sorafenib maintenance versus control*

*Adapted from Xuan et al.1
Conclusion
This long-term follow-up study demonstrated the benefits of sorafenib maintenance in patients with FLT3-mutated AML undergoing allo-HSCT. It shows that OS, LFS, and GRFS were greatly improved, leading to long-term survival in this population. The authors commented that 5 years was the longest duration of follow-up for sorafenib maintenance in patients with FLT3-mutated AML in a transplant setting. However, the study’s limitations should be considered when interpreting the findings. This study was an extension of a post hoc analyses with small sample sizes in some subgroups. The study included patients younger than 60 years who received myeloablative conditioning, limiting the generalizability of the findings.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



