All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Imetelstat in patients with lower-risk MDS: Updates from the IMerge trial
In patients with lower-risk myelodysplastic neoplasms (LR-MDS) who are red blood cell (RBC) transfusion-dependent, there remains a need for novel therapies following relapsed or refractory disease or failure of erythropoiesis-stimulating agents.1 Imetelstat is a first-in-class telomerase inhibitor, targeting the ribonucleic acid template of telomerase.1 The MDS Hub has previously reported on the efficacy of imetelstat in patients with highly transfusion-dependent MDS and LR‑MDS from the phase II portion of the IMerge trial (NCT02598661).
We are pleased to provide a summary of three oral presentations from the European Hematology Association (EHA) 2023 Hybrid Congress, relating to the phase III results from the IMerge trial; Uwe Platzbecker1 presented efficacy and safety data, Valeria Santini2 covered disease activity, and Chapuis3 discussed biological pathways underlying the clinical response to imetelstat. These findings were also outlined by Zeidan4 at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting.
IMerge study design and patient baseline characteristics
Patients with LR-MDS were randomized to receive imetelstat or placebo (Figure 1).1,2,4
Figure 1. Study design and treatment schema of the phase III portion of the IMerge trial*
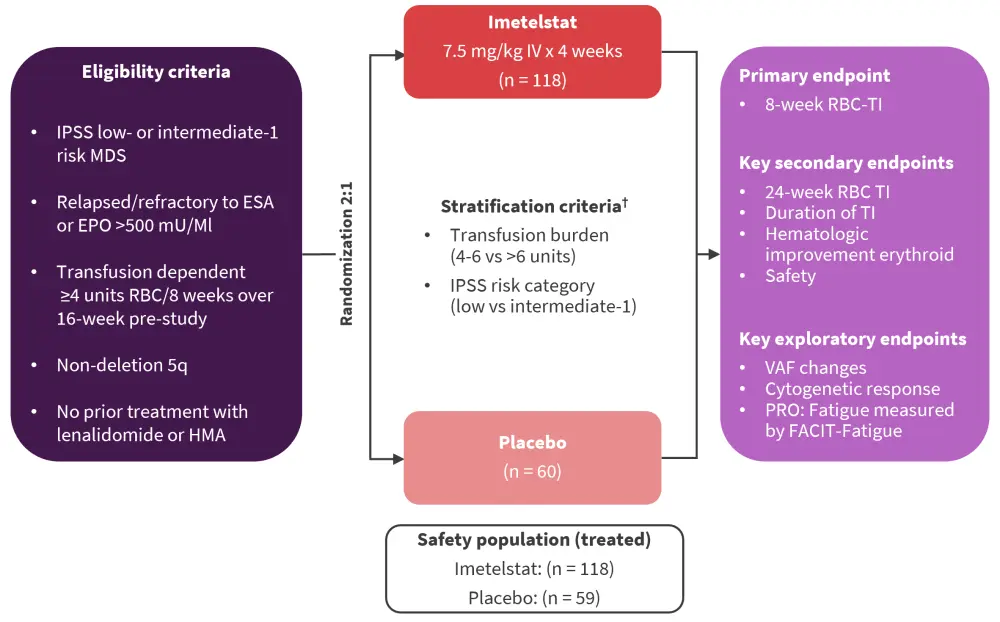
EPO, erythropoietin; ESA, erythropoiesis-stimulating agent; FACIT-fatigue, functional assessment of chronic illness therapy-fatigue; HMA, hypomethylating agent; IPSS, International Prognostic Scoring System; ITT, intent-to-treat; IV, intravenous; MDS, myelodysplastic neoplasm; RBC, red blood cell; TI, transfusion independence; VAF, variant allele frequency.
*Adapted from Platzbecker,1 Santini,2 and Zeidan.4
†Supportive care, including RBC and platelet transfusions, myeloid growth factors (e.g., G-CSF), and iron chelation therapy administered at the investigator’s discretion.
Ten patients were included in the subgroup analysis to identify biological pathways associated with clinical response of imetelstat, six of whom were transfusion independent (TI) responders and four were TI non-responders.3 Bone marrow (BM), mononuclear cell transcriptome, peripheral blood immune cell landscape, and plasma cytokines were analyzed.3
At the data cut-off date of October 13, 2022, 178 patients with LR-MDS were included, who were heavily transfusion dependent in both the imetelstat and placebo groups.1,2,4 Baseline characteristics are shown in Table 1.
Table 1. Baseline characteristics*
|
ESA, erythropoiesis-stimulating agent; Hgb, hemoglobin; IPSS, International Prognostic Scoring System; IPSS-M, IPSS-Molecular; IPSS-R, IPSS-Revised; RBC, red blood cell; RS, ring sideroblast; TI, transfusion independence; sEPO, serum erythropoietin; WHO, World Health Organization. |
||
|
Characteristic, % (unless stated otherwise) |
Imetelstat |
Placebo |
|---|---|---|
|
Median age (range), years |
72 (44–87) |
73 (39–85) |
|
Sex, male |
60 |
67 |
|
Median time since diagnosis (range), years |
3.5 (0.1–26.7) |
2.8 (0.2–25.7) |
|
WHO classification |
|
|
|
RS positive |
62 |
62 |
|
RS negative |
37 |
38 |
|
IPSS risk category |
|
|
|
Low |
68 |
65 |
|
Intermediate-1 |
32 |
35 |
|
IPSS-R risk category |
|
|
|
Low |
73.7 |
76.7 |
|
Intermediate-1 |
16.9 |
13.3 |
|
IPSS-M risk category |
|
|
|
Low |
63.1 |
63.5 |
|
Moderate low |
21.4 |
19.2 |
|
Cytogenetic abnormality based on central laboratory review |
22 |
22 |
|
Median pretreatment Hgb (range), g/dL |
7.9 (5.3–10.1) |
7.8 (6.1–9.2) |
|
Median prior RBC transfusion burden (range), RBC units/8 weeks |
6 (4–33) |
6 (4–13) |
|
Prior transfusion burden |
|
|
|
≥4 to ≤6 units/8 weeks |
53 |
55 |
|
>6 units/8 weeks |
48 |
45 |
|
Median sEPO (range), mU/mL |
174.9 (6.0–4,460.0) |
277 (16.9–5,514.0) |
|
sEPO level |
|
|
|
≤500 mU/mL |
74 |
60 |
|
>500 mU/mL |
22 |
37 |
|
Prior ESA |
92 |
87 |
|
Prior luspatercept |
6 |
7 |
Imetelstat increased RBC-TI up to 52 weeks1,4
At a median follow-up of 18 months, 77.1% vs 76.3% of patients in the imetelstat and placebo group, respectively, discontinued treatment due to lack of efficacy (23.8% vs 42.4%), adverse events (AEs) (16.1% vs 0%), and loss of response (14.4% vs 1.7%).
RBC-TI was consistently higher with imetelstat compared with placebo at 8, 16, 24, and 52 weeks (Figure 2).
- The median duration of RBC-TI was 51.6 weeks vs 13.3 weeks in the imetelstat and placebo groups, respectively (hazard ratio [HR], 0.23; p < 0.001).
- 8-week RBC-TI rate was also greater with imetelstat versus placebo across subgroups, including those with ring sideroblasts (RS) and those transfused with >6 units/8 weeks.
- The 24-week RBC-TI seen with imetelstat was irrespective of baseline mutation status (p < 0.001), baseline telomerase activity (p = 0.074), telomerase length (0.001), and human telomerase reverse transcriptase (p = 0.042).
In addition, patients treated with imetelstat demonstrated:
- An overall durability of RBC-TI across all subgroups at 8 weeks (HR, 0.23; p < 0.001) and 24 weeks (p < 0.001).
- A greater reduction in mean RBC transfusion units over time compared with placebo (p = 0.042).
- Greater hematologic improvement-erythroid rates versus placebo (50% vs 8%, p < 0.001). This was also noted for major (30.9% vs 0%, p < 0.001) and minor (44.3% vs 9.5%, p < 0.001) hematologic improvement-erythroid rates.
Figure 2. RBC-TI over time with imetelstat*
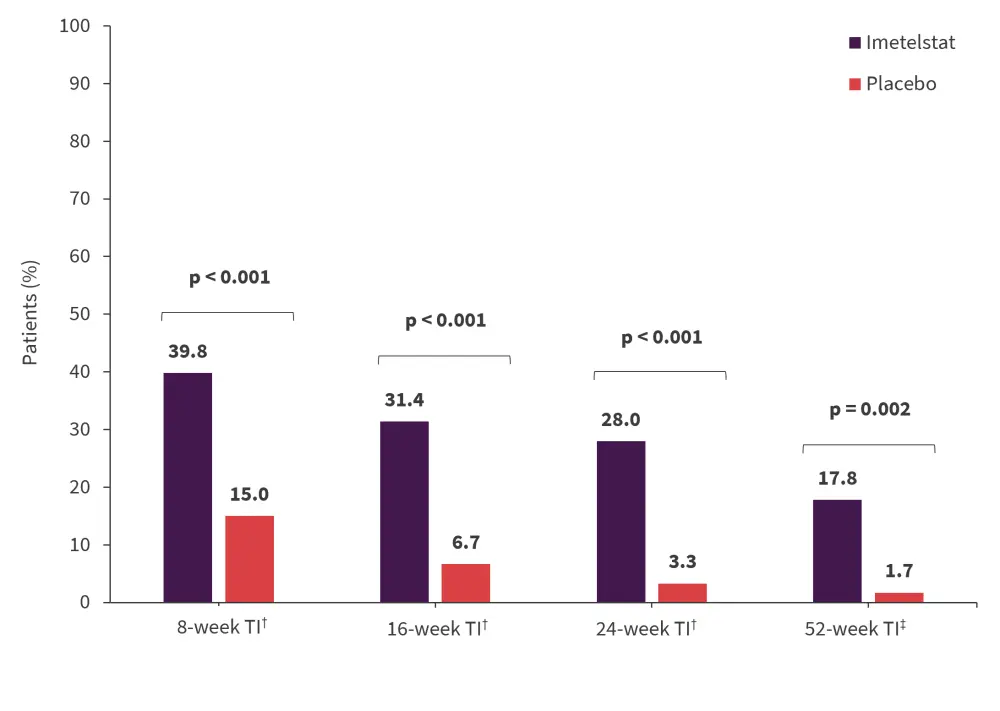
RBC, red blood cell; TI, transfusion independence.
*Adapted from Platzbecker1 and Zeidan.4
†Data cut-off: October 13, 2022.
‡Data cut off: January 13, 2023.
The most common safety signals were hematologic1,4
- The most frequently reported Grade 3–4 AEs were thrombocytopenia (62%) and neutropenia (68%) in the imetelstat group, and thrombocytopenia (8%) and anemia (7%) in the placebo group; Grade 3 febrile neutropenia was observed in one patient in the imetelstat group.
- The median duration of thrombocytopenia was 1.4 weeks (range, 0.1–12.6 weeks) and 2 weeks (range, 0.3–11.6 weeks) in the imetelstat and placebo groups, respectively.
- The median duration of neutropenia was 1.9 weeks (range, 0.0–15.9 weeks) and 2.2 weeks (range, 1.0–4.6 weeks) in the imetelstat and placebo groups, respectively.
- Both thrombocytopenia and neutropenia were resolved in >80% of patients in the imetelstat group within 4 weeks.
- No fatal hematologic AEs were observed in either treatment group.
- Fewer than 15% of patients discontinued treatment due to treatment-emergent AEs; however, the median time to treatment discontinuation was 21.1 weeks (range, 2.3–44 weeks).
Imetelstat induces cytogenetic response and increases TI duration2,4
Cytogenetic complete or partial response was achieved by 35% (imetelstat) and 15% (placebo) of patients with cytogenetic abnormalities at baseline, and 8-week RBC-TI was attained by 89% (imetelstat) and 50% (placebo) of patients with a cytogenetic response.
Compared with placebo, patients treated with imetelstat showed:
- ≥50% reduction in central BM RS (40.8% vs 9.7%); interestingly, patients in the imetelstat group achieving ≥50% reduction in central BM RS also showed a higher rate of RBC-TI.
- A greater reduction in variant allele frequency (VAF) of SF3B1 (p <0.001), TET2 (p = 0.032), DNMT3A (p = 0.019), and ASXL1 (p = 0.146) genes.
- A higher proportion with ≥50% VAF reduction in SF3B1 (29.5% vs 2.6%, p = 0.001), TET2 (34.3% vs 8.3%), DNMT3A (11.1% vs 0%), and ASXL1 (40% vs 16.7%); the reduction in SF3B1 VAF was maintained over time in the imetelstat group.
In those patients who received imetelstat, the reductions noted in VAF in SF3B1, TET2 and DNMT3A genes correlated with clinical outcomes:
- Longer duration of RBC-TI: SF3B1 (p < 0.001), TET2 (p < 0.001), and DNMT3A (p = 0.019)
- Increased hemoglobin levels: SF3B1 (p < 0.001), TET2 (p < 0.001), and DNMT3A (p = 0.013)
In the imetelstat group, RBC-TI also correlated with reductions in RS-positive cells, cytogenetic responses, and VAF in commonly mutated genes at 8 weeks and 24 weeks (Figure 3).
- Those who attained ≥50% reduction in SF3B1 had a higher 1-year RBC-TI compared with those in the placebo group (47.8% vs 5.5%, p < 0.001).
- Similarly, patients who attained ≥50% reduction in TET2 had a higher 1-year RBC-TI compared with those in the placebo group (50% vs 8.7%, p = 0.011).
Figure 3. Correlation of RBC-TI with reductions in RS, VAF, and cytogenetic responses at A 8 weeks and B 24 weeks*
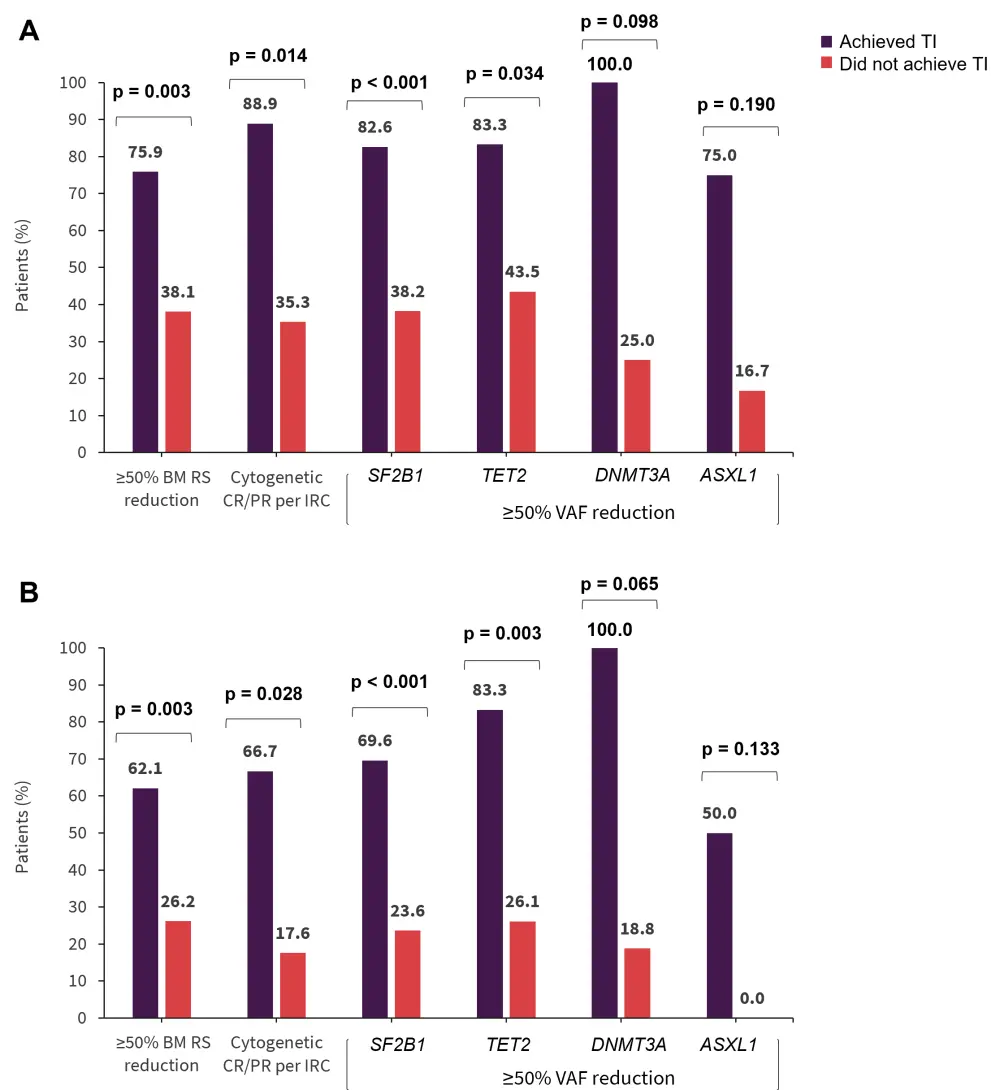
BM, bone marrow; CR, complete response; IRC, independent review committee; PR, partial response; RBC, red blood cell; RS, ring sideroblast; TI, transfusion independence; VAF, variants allele frequency.
*Adapted from Santini.2
Imetelstat modulates the immune landscape in LR-MDS3
Of the ten patients included, six patients had International Prognostic Scoring System (IPSS) LR-MDS and four had intermediate-1 MDS. A total of 1,185 differentially expressed genes were identified; 1,150 were downregulated and 35 were upregulated.
In patients who achieved TI:
- A higher number of differentially expressed genes were expressed in patients achieving TI versus those who did not achieve TI (p = 0.05).
- Innate and adaptive immune responses were suppressed and pro-inflammatory interleukin (IL)-8 or CXCL9 chemokine levels were lower.
- Treatment with imetelstat for 4–7 months resulted in:
- Upregulation of pathways involved in T-cell activation and B-cell proliferation and downregulation of IL-6 and IL-1β production.
- Reduced proportion of human leukocyte antigen-DR monocytes and increased pro-inflammatory monocyte CD16+ levels.
- Increased levels of CD8+ terminal effector T cells and B cells
- Increased levels of tumor necrosis factor α, CXCL9, IL-17F, and IL-6, but decreased S100A8/A9 heterodimer (after two cycles of treatment).
Conclusion
These findings demonstrate the clinically meaningful efficacy of imetelstat in patients with heavily transfusion-dependent LR-MDS. Patients treated with imetelstat achieved higher cytogenetic response rates and consequently 8-week RBC TI. The safety profile of imetelstat was also consistent with previous studies.1 There was a sustained reduction in SF3B1 VAF over time and greater reduction in multiple genes.1,2 The findings suggest that imetelstat may impact the biology of LR-MDS and potentially lead to change in the course of the disease by reducing or removing malignant clones and improving inefficient erythropoiesis.2 Imetelstat was associated with induction of an adaptive immune response, indicating that reconfiguration of immune cells may result in hematopoietic activity. Further studies are needed to establish the contribution of immunomodulation to the erythroid response.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

