All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
The impact of IDH2 mutant allele (R140/R172) on survival outcomes with enasidenib: An update from the IDHENTIFY trial
Mutations in the isocitrate dehydrogenase 2 (IDH2) gene occur in around 8–19% of patients with acute myeloid leukemia (AML).1 Enasidenib, an oral inhibitor of IDH2, is the only U.S. Food and Drug Administration (FDA)-approved therapy for this group of patients with relapsed or refractory (R/R) disease. We have previously outlined the IDHENTIFY trial of enasidenib versus conventional care regimens (CCR) in elderly patients with late-stage IDH2-mutated R/R AML (NCT02577406), which failed to meet its primary endpoint of increased overall survival. During the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, De Botton, et al.1 reported on the safety and efficacy analyses from the IDHENTIFY trial for patient subgroups defined by the IDH2 gene point mutations R140K and R172K. We summarize the key points below.
Co-mutational profile
Of the 319 patients enrolled, 229 (72%) had the IDH2R140 mutation, and 88 (28%) had the IDH2R172 mutation. IDH2 variable allele frequencies at baseline were highly variable but similar between treatment arms and IDH2 variants.
In the IDH2R140 subgroup, the median number of baseline co-mutations was 5 (range, 1–11), with RUNX1 (59%) and SRSF2 (59%) being the most common. Moreover, this subgroup of patients was enriched with generally poor-risk mutations, including FLT3 (either the internal tandem duplication, or in the tyrosine kinase domain), NRAS, and RUNX1. In the IDH2R172 subgroup, DNMT3A (57%) and RUNX1 (43%) were the most frequent co-mutations, with enrichment of both DNMT3A and TP53 compared to the IDH2R140 subgroup. In addition, the median 2-hydroxyglutarate (2-HG) levels at baseline were similar between the treatment arms enasidenib (770.5 ng/mL) and CCR (791.8 ng/mL), and the mutant IDH2 variant subgroups IDH2R140 (774.5 ng/mL) and IDH2R172 (872.0 ng/mL).
Clinical responses
The overall response rate, complete remission rate, red blood cell transfusion independence, and hematologic improvement were all increased in patients treated with enasidenib versus CCR, in both subgroups (Figure 1).
Figure 1. Response rates for patients with IDH2R140 or IDH2R172 mutations treated with enasidenib or conventional care regimens*
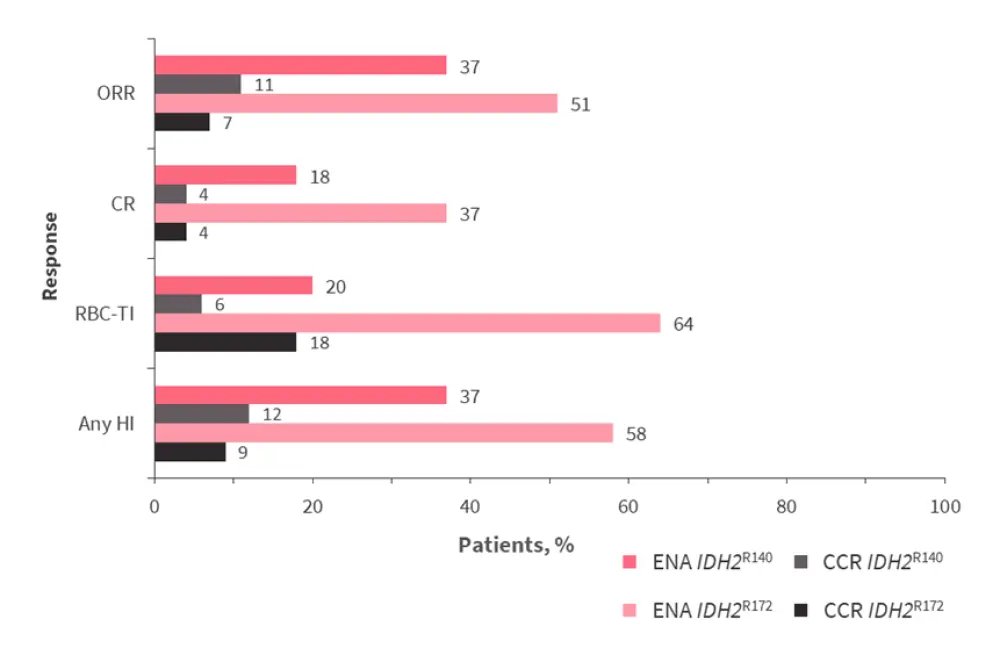
CCR, conventional care regimens; CR, complete remission; ENA, enasidenib; HI, hematologic improvement; ORR, overall response rate; RBC-TI, red blood cell transfusion independence.
*Adapted from De Botton, et al.1
Survival outcomes for patients with IDH2R140 and IDH2R172 mutations are shown in Table 1. Treatment with enasidenib conferred a significant survival benefit compared to CCR in patients with IDH2R172. In patients with IDH2R140, although time-to-treatment failure was improved with enasidenib treatment, the overall survival was similar between arms.
Table 1. Survival rates for patients with either IDH2R140 or IDH2R172 treated with enasidenib or conventional care regimens*
|
CCR, conventional care regimens; CI, confidence interval; HR, hazard ratio; EFS, event-free survival; ENA, enasidenib; OS, overall survival; TTF, time-to-treatment failure. |
||||||
|
Survival measure, months |
IDH2R140 (n = 229) |
IDH2R172 (n = 88) |
||||
|---|---|---|---|---|---|---|
|
ENA |
CCR |
HR (95% CI); |
ENA |
CCR |
HR (95% CI); |
|
|
OS |
5.7 |
5.7 |
0.93 |
14.6 |
7.8 |
0.59 |
|
EFS |
3.9 |
1.9 |
0.77 |
10.1 |
2.7 |
0.47 |
|
TTF |
4.3 |
1.6 |
0.63 |
7.5 |
2.2 |
0.30 |
Safety
The median duration of treatment was substantially longer with enasidenib compared to CCR (142 days versus 36 days, respectively). The rates of common adverse events were comparable between patients in the IDH2R140 and IDH2R172 subgroups in both treatment arms (Table 2). The rates of enasidenib-related increases in bilirubin levels (IDH2R140, 21%; IDH2R172, 17%) and IDH differentiation syndrome (IDH2R140, 14%; IDH2R172, 14%) were comparable between treatment arms.
Table 2. Grade ≥3 treatment-related adverse events occurring in ≥10% of patients*
|
CCR, conventional care regimens; ENA, enasidenib. |
||||
|
Preferred term, % |
IDH2R140 |
IDH2R172 |
||
|---|---|---|---|---|
|
ENA (n = 115) |
CCR (n = 101) |
ENA (n = 42) |
CCR (n = 40) |
|
|
Blood bilirubin increase |
10 |
0 |
2 |
0 |
|
Febrile neutropenia |
2 |
12 |
5 |
13 |
|
Neutropenia |
3 |
10 |
12 |
13 |
|
Thrombocytopenia |
7 |
8 |
19 |
10 |
Conclusion
The IDH2R140 variant was associated with poor-risk mutations and a significantly greater baseline mutational burden versus IDH2R172. Enasidenib improved the overall response rate, complete remission, red blood cell transfusion independence, and hematologic improvement compared to CCR in both mutational subgroups, with similar safety profiles, also observed between treatments. The survival benefits provided by enasidenib were more evident in patients with the IDH2R172 mutation than IDH2R140. Additional research is required to investigate the influence of the distinct IDH2R140/IDH2R172 co-mutational profiles on treatment outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

