All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
The funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
HOVON 116 trial: Epigenetic therapy with panobinostat and/or decitabine after allo-SCT for poor-risk AML
Allogeneic stem cell transplantation (allo-SCT) is the current standard of care for patients with poor-risk acute myeloid leukemia (AML).1 Allo-SCT aims at establishing remission by exploiting the graft-versus-leukemia (GvL) effect. Nevertheless, especially in poor-risk patients, disease relapse following allo-SCT is still one of the main reasons of failure in AML.1 Thus, there is a great need for treatments that enhance the GvL effect when combined with allo-SCT, to lead to better outcomes. For this reason, Burak Kalin et al.1 investigated in the phase I/II trial, HOVON 116, the feasibility and preliminary efficacy of epigenetic therapy with panobinostat (PNB) and/or decitabine (DAC) in poor-risk AML patients. The results of this study were published in Blood Advances and are summarized below.
Study design
- Prospective, multicenter, phase I/II feasibility trial
- The study included poor and very poor-risk patients, aged 18–70, with AML or refractory anemia with excess blasts, prior to the start of the second induction chemotherapy cycle. The treatment intention for responding patients was to receive allo-SCT 6–8 weeks after the second induction chemotherapy cycle
- In the phase I part of the study, three dosing schedules were explored in 29 very-poor-risk patients with AML:
- Dose 1 (PNB): 20 mg panobinostat at Days 1, 4, 8, and 11, of 4-week cycles
- Dose 2 (PNB/DAC20): 20 mg panobinostat monotherapy at Days 1, 4, 8, and 11 in combination with 20 mg/m2 decitabine at Days 1–3, of 4-week cycles
- Dose 3 (PNB/DAC10): 20 mg panobinostat monotherapy at Days 1, 4, 8, and 11 in combination with 10 mg/m2 decitabine at Days 1–3, of 4-week cycles
- The primary endpoint of phase I was the feasibility of these three dosing schedules based on their dose-limiting toxicities (DLTs) in the first treatment cycle
- In the phase II part, only the most feasible, PNB and PNB/DAC10 dosages were further assessed, primarily for their safety, and secondarily for their efficacy
- The treatment scheme of the study is shown below in Figure 1
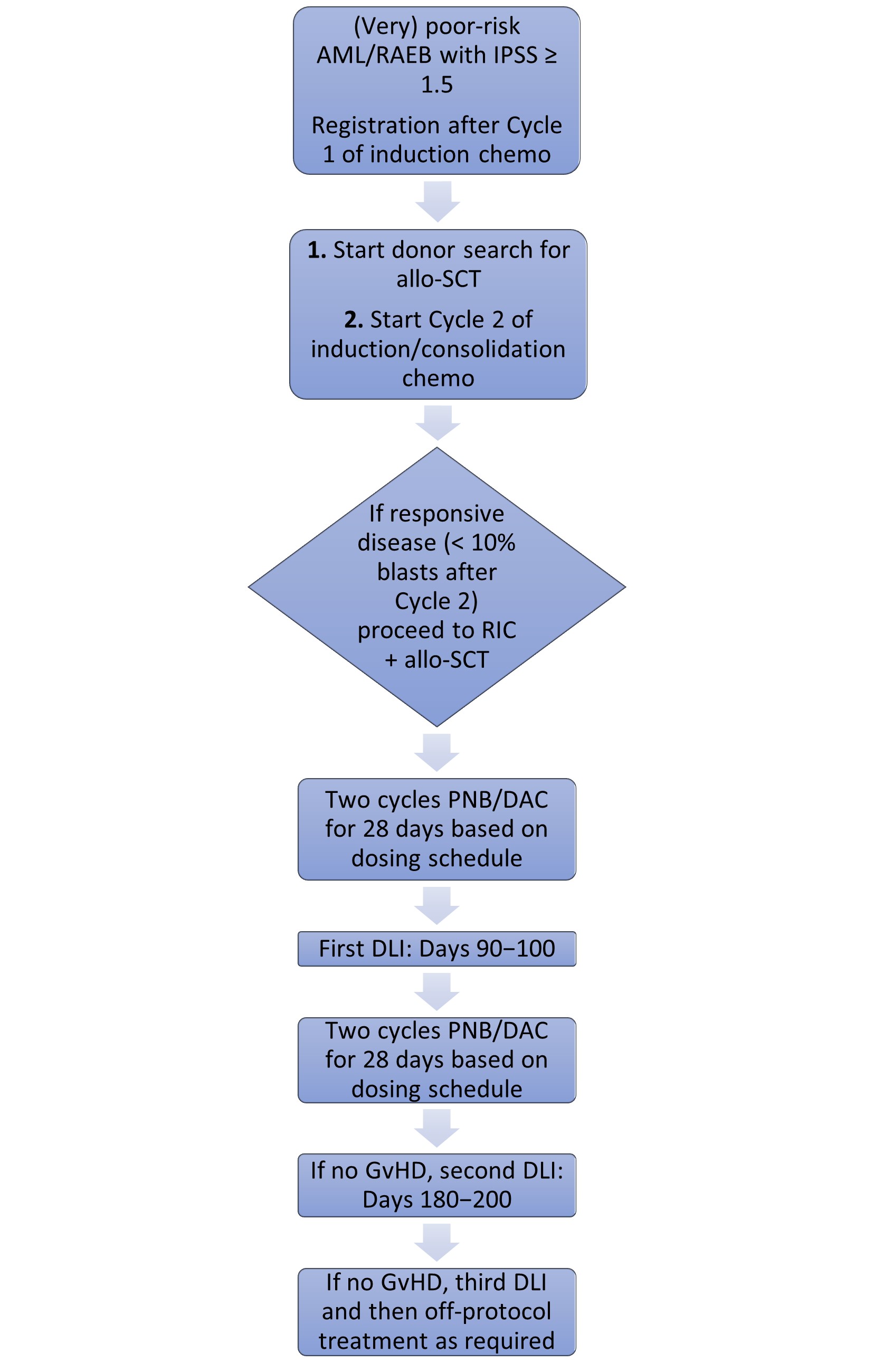
Results
A total of 110 patients who were registered in the study underwent allo-SCT. The key patient baseline characteristics are shown below in Table 1. Briefly, the median age was 59 years, the majority of patients had a World Health Organization (WHO) performance status of 0–1 (83%) and a very poor HOVON/SAKK risk classification (70%)
Table 1. Patient baseline characteristics in HOVON 1161
|
CR, complete response; CRi, CR with incomplete hematological recovery; EVI1, ecotropic viral integration 1; FC, flow cytometry; HOVON, Dutch-Belgian Cooperative Trial Group for Hematology Oncology; MK, monosomal karyotype; MRD, measurable residual disease; PR, partial response; SAKK, Swiss Group for Clinical Cancer Research; WHO, World Health Organization |
|
|
Characteristic |
Patient cohort (N = 110) |
|---|---|
|
Median age (range), years |
59 (18–71) |
|
Male patients, % |
61 |
|
WHO performance status, % 0 1 2 Unknown |
46 37 10 6 |
|
Response to induction Cycle 1, % CR/CRi PR Refractory |
61 7 32 |
|
Response to induction Cycle 2, % CR/CRi PR |
96 4 |
|
MRD FC (n = 74), % Positive Negative |
2 72 |
|
HOVON/SAKK risk group, % Poor Very poor MK EVI1 |
30 70 19 25 |
Phase I part
- DLTs were experienced by:
- 1/9 patients in the PNB group
- 1/10 patients in the PNB/DAC10 group
- 4/10 patients in the PNB/DAC20 group
- Due to the high proportion of prolonged cytopenia DLTs observed in the PNB/DAC20 group, this was not further evaluated in the phase II part of the study
Phase II part
- Of the 110 patients who underwent allo-SCT, 87 received their first treatment cycle with either PNB or PNB/DAC10
- In total, 55% of patients were eligible for donor lymphocyte infusion (DLI) within 115 days, and 84% of patients who received a second PNB or PNB/DAC10 cycle received their first DLI
- Second and third DLIs were successfully given to 40 and 25 patients, respectively
- The main reasons for PNB or PNB/DAC10 withdrawal were:
- Graft-versus-host disease (GvHD; n = 11)
- Thrombocytopenia (n = 3)
- Renal dysfunction (n = 3)
- Death (n = 2)
- Disease progression (n = 2)
- Liver dysfunction (n = 1)
- Start of Cycle 1 after Day 35 post allo-SCT (n = 1)
- Treatment-related adverse events (TEAEs) by system are shown below in Table 2. Grade 3–4 adverse events (AEs) due to PNB/DAC treatment were seen in 26% of patients
- In general, TEAEs were easily manageable with quick recovery following treatment discontinuation
Table 2. Treatment-related toxicities by system in the HOVON 1161
|
DAC, decitabine; PNB, Panobinostat. |
||||
|
Treatment-related toxicities |
PNB monotherapy (n = 39), % |
PNB/DAC10 (n = 35), % |
||
|---|---|---|---|---|
|
Grade 2 |
Grade 3 and above |
Grade 2 |
Grade 3 and above |
|
|
Blood and bone marrow |
2 |
— |
— |
— |
|
Gastrointestinal |
4 |
4 |
6 |
4 |
|
Constitutional symptoms |
4 |
2 |
— |
— |
|
Infections |
7 |
— |
— |
2 |
|
Metabolic/laboratory |
— |
4 |
— |
4 |
|
Eye |
2 |
— |
— |
— |
|
Nervous system |
— |
2 |
— |
2 |
|
Skin and subcutaneous tissue |
2 |
— |
— |
— |
- With regard to preliminary efficacy results, these are shown in Table 3, and these account for all patients who underwent allo-SCT. When looking at the different dosing schedules, there was no difference in the 2-year cumulative relapse incidence between the PNB (24%) and PNB/DAC10 groups (46%; p = 0.29)
- When stratifying patients by HOVON risk, very poor-risk patients with AML had a lower 2-year OS rate than those with poor-risk AML (46% vs 63%)
- In the total cohort, Grade 3–4 and Grade 2–4 acute GvHD rates at 6 months were 5% and 23%, respectively. At 12 months, moderate-to-severe chronic GvHD had developed in 22% of patients
- None of the patients required systemic GvHD therapy or experienced relapse at the time of follow-up
Table 3. Efficacy outcomes from HOVON 1161
|
Allo-SCT, allogeneic stem cell transplantation; CIR, cumulative incidence of relapse; NRM, non-relapse mortality; OS, overall survival; PFS, progression-free survival |
|
|
Outcome |
Patients who underwent allo-SCT, % (N = 110) |
|---|---|
|
2-year CIR |
35 |
|
2-year NRM |
16 |
|
2-year PFS |
49 |
|
2-year OS |
50 |
Conclusion
The results of this phase I/II trial showed that panobinostat monotherapy or in combination with 10 mg/m2 decitabine are feasible and tolerable epigenetic regimens for patients with poor-risk AML, who have undergone allo-SCT. Data did not indicate a synergistic or additive effect of panobinostat and decitabine. Due to the preliminary nature of these results, further large-scale prospective trials are needed to assess the efficacy of these post allo-SCT epigenetic therapies for the treatment of AML patients with poor outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

