All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Gilteritinib plus azacitidine in patients with FLT3-mutated AML: updated results from the LACEWING trial
A mutation in the FMS-like tyrosine kinase 3 (FLT3) gene, a recurrent genetic abnormality in acute myeloid leukemia (AML), confers a poor prognostic outcome for patients. Typically, patients with FLT3-mutated AML are treated with intensive chemotherapy, but if patients are ineligible, alternative options are limited.1
Gilteritinib is an oral, small-molecule, second-generation FLT3 inhibitor approved by the U.S. Food and Drug Administration and the European Commission for the treatment of relapsed/refractory AML. The phase III LACEWING (NCT02752035) trial, previously covered by the AML Hub, assessed the efficacy and safety of gilteritinib plus azacitidine versus azacitidine monotherapy in adults with newly diagnosed FLT3-mutated AML. The latest data from this trial were published by Wang, et al., in Blood in 2022, and we are pleased to summarize them below.1
Methods
The LACEWING study was a phase III, randomized, open-label trial spanning 185 centers in North America, Europe, and Asia/Pacific. The primary outcome was overall survival (OS), and secondary outcomes included event-free survival (EFS), complete remission (CR) rates, treatment failure and death. Eligible patients were
- aged ≥18 years;
- previously untreated for AML;
- positive for an FLT3 (internal tandem duplication [ITD] and/or tyrosine kinase domain [TKD]) mutation; and
- ineligible for intensive induction chemotherapy.
Figure 1. Eligibility assessment and randomization of patients*
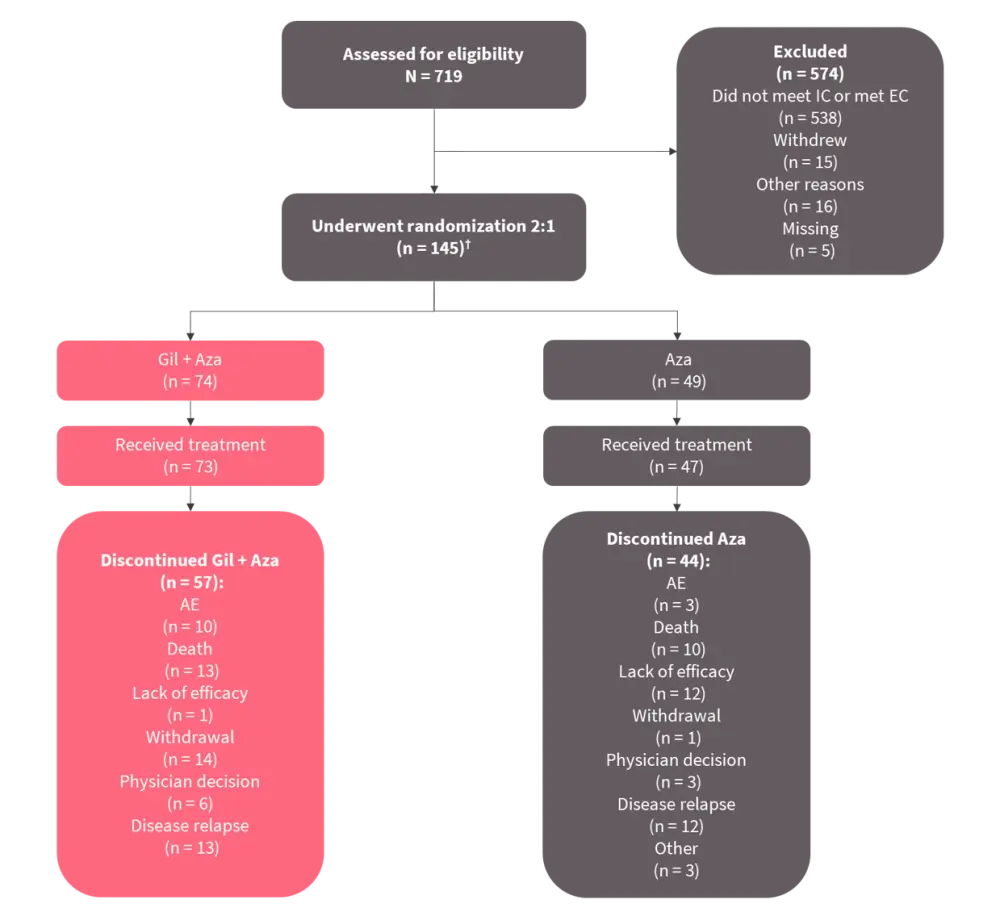
AE, adverse event; AZA, azacitidine; EC, exclusion criteria; GIL, gilteritinib; IC, inclusion criteria.
*Adapted from Wang, et al.1
†Initially, this study included a gilteritinib monotherapy arm (n = 22), but this arm was removed due to changes in the preferred treatment for this patient population.
Results
Patient characteristics
Enrollment in this study was discontinued for futility based on OS results. As of August 26, 2020, 123 patients were randomized (2:1) to receive either gilteritinib plus azacitidine or azacitidine (Table 1). Nearly three-quarters of patients in each treatment group were aged ≥75 years.
Table 1. Patient characteristics*
|
AR, allelic ratio; Aza, azacitidine; BSA, body surface area; ECOG, Eastern Cooperative Oncology Group; FLT3, FMS-like tyrosine kinase 3; Gil, gilteritinib; ITD, internal tandem duplication; PS, performance status; SD, standard deviation; TKD, tyrosine kinase domain. |
||
|
Characteristic, % (unless otherwise stated) |
Gil + Aza |
Aza |
|---|---|---|
|
Male |
57 |
57 |
|
Age, years |
|
|
|
Median (min–max) |
78 (59–99) |
76 (61–88) |
|
Baseline ECOG PS |
|
|
|
0–1 |
51 |
65 |
|
≥2 |
47 |
33 |
|
Baseline FLT3 mutation type |
|
|
|
ITD only |
78 |
82 |
|
TKD (D835/I836) only |
19 |
14 |
|
ITD with TKD (D835/I836) |
3 |
4 |
|
Baseline FLT3 mutation status |
|
|
|
ITD AR <0.5 |
34 |
37 |
|
ITD AR ≥0.5 |
47 |
49 |
|
TKD |
19 |
14 |
|
Cytogenic risk |
|
|
|
Favorable |
3 |
0 |
|
Intermediate |
69 |
74 |
|
Unfavorable |
11 |
10 |
|
Other (unknown/missing) |
18 |
16 |
Overall Survival
Median OS was 9.82 months for the gilteritinib plus azacitidine arm and 8.87 months for the azacitidine only arm (hazard ratio [HR], 0.916 [95% confidence interval [CI], 0.529–1.585]; p = 0.753), with a median follow-up time of 9.76 months and 17.97 months respectively. A survival benefit with gilteritinib plus azacitidine versus azacitidine monotherapy was observed in the following patient subgroups:
- Eastern Cooperative Oncology Group performance status of 0–1 (13.17 vs 11.89 months; HR, 0.811 [95% CI, 0.409–1.608])
- FLT3-ITD allelic ratio ≥0.5 (10.68 vs 4.34 months; HR 0.580 [95% CI, 0.285 – 1.182])
However, patients with FLT3-TKD mutations who received gilteritinib plus azacitidine had a shorter OS than those who received azacitidine only (4.86 vs 11.89 months; HR, 2.504 [95% CI, 0.746–8.411]).
Figure 2. Comparison of complete remission rates between treatment groups*
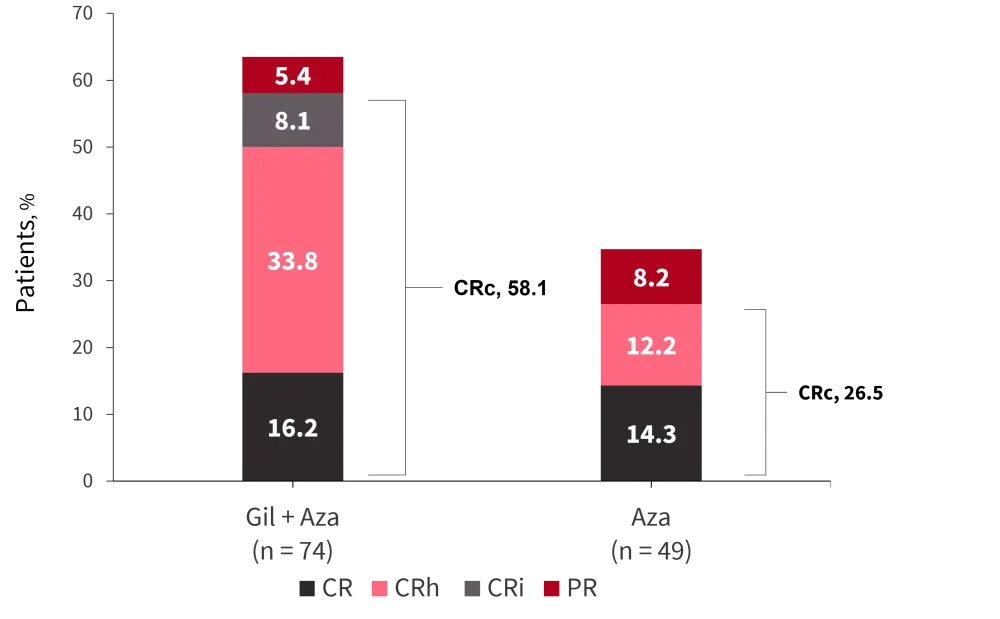
Aza, azacitidine; CR, complete remission; CRc, composite complete remission; CRh, complete remission with incomplete hematological recovery; CRi, complete remission with incomplete platelet recovery; Gil, gilteritinib.
*Adapted from Wang, et al.1
Event-free survival
Median EFS was 0.03 months in both treatment arms (HR, 0.925 [95% CI, 0.592–1.444]; p = 0.839). However, sensitivity analysis of EFS based on composite complete remission (CRc) found median EFS was improved in the gilteritinib plus azacitidine group (4.53 months) compared with the azacitidine group (0.03 months).
Response rate
- CR rates were analogous between the two arms (gilteritinib plus azacitidine, 16.2%; azacitidine, 14.3%).
- Median time to CR was 116.5 days for gilteritinib plus azacitidine and 95 days for azacitidine.
- CRc rates were higher in patients who received gilteritinib plus azacitidine at 58.1%, compared with 26.5% for azacitidine (Figure 2.; p < 0.001).
- Within the FLT3 subgroups, CRc rates were highest in patients who received gilteritinib plus azacitidine with FLT3-ITD allelic ratio ≥0.5 (71.4% vs 20.8%; p = 0.003).
- The median time to CRc was equal for both treatment arms at 57 days.
- Of the 12 patients who achieved CR in the gilteritinib plus azacitidine treatment arm, ten remained in CR, while the median CR duration was 8.57 months for the seven patients who achieved CR in the azacitidine arm.
Safety
Adverse event (AE) rates were 100% and 95.7% in the gilteritinib plus azacitidine and azacitidine treatment arms, respectively (Table 2). The AEs leading to death were considered treatment related in four patients in each group. Gastrointestinal hemorrhage and QT prolongation were identified as AEs of special interest, and occurred in 12.3% and 13.7% of patients in the gilteritinib plus azacitidine treatment group, and in 6.4% and 0% of patients in the azacitidine treatment group (Table 2).
Table 2. Adverse events in each treatment group*
|
AZA, azacitidine; GIL, gilteritinib. |
||
|
Adverse event, % |
GIL+AZA |
AZA |
|---|---|---|
|
Grade ≥3 |
Grade ≥3 |
|
|
Overall |
95.9 |
89.4 |
|
Pyrexia |
9.6 |
0 |
|
Diarrhea |
6.8 |
0 |
|
Febrile neutropenia |
35.6 |
19.1 |
|
Anemia |
24.7 |
27.7 |
|
Thrombocytopenia |
27.4 |
19.1 |
|
Pneumonia |
20.5 |
17 |
|
Neutropenia |
21.9 |
21.3 |
|
Aspartate aminotransferase increased |
5.5 |
0 |
|
Vomiting |
2.7 |
0 |
|
Asthenia |
6.8 |
0 |
|
Hypokalemia |
8.2 |
8.5 |
|
Decreased appetite |
4.1 |
2.1 |
|
Neutrophil count decreased |
19.2 |
8.5 |
|
Platelet count decreased |
17.8 |
19.1 |
|
Hyponatremia |
12.3 |
2.1 |
|
Sepsis |
5.5 |
10.6 |
Pharmacokinetic parameters
There were no substantial differences in gilteritinib trough concentrations at steady state (Ctrough) between gilteritinib plus azacitidine and gilteritinib monotherapy (before removal). Low Ctrough was associated with longer OS and longer median time to discontinuation versus high Ctrough.
Conclusion
Although survival was similar between patients treated with or without gilteritinib alongside azacitidine, in those with high FLT3-ITD allelic burden (allelic ratio ≥0.5), gilteritinib improved OS by 6.3 months. Furthermore, CRc rates were significantly higher in the gilteritinib plus azacitidine arm, although CR rates were similar. The safety profile of gilteritinib in combination with azacitidine was consistent with the profiles of each individual therapy.
Overall, the LACEWING trial demonstrated the safety and favorable clinical activity of gilteritinib plus azacitidine in previously untreated patients with FLT3-mutated AML who are considered unfit for intensive induction chemotherapy. In particular, patients with FLT3-ITD AML and a high allelic burden benefitted most from gilteritinib plus azacitidine, which should be investigated further.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




