All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
FLAG-IDA + venetoclax for the treatment of patients with newly diagnosed or R/R AML
Featured:
FMS-like tyrosine kinase 3 (FLT3) mutations are the most common genetic abnormality found in acute myeloid leukemia (AML), being present in 25−30% of cases. The two most common forms of this mutation are internal tandem repeat and tyrosine kinase domain point mutation. Patients with FLT3 mutations are classified as high-risk and show increased risk of relapse.1
Midostaurin, a FLT3 inhibitor, is one of the most frequently used agents for treating FLT3-mutated AML. However, most other FLT3 inhibitors have produced only short-lived responses and there remains a need to find alternative treatments. Venetoclax, a Bcl-2 inhibitor, in combination with hypomethylating agents has produced good responses in treatment-naive frail patients and patients with relapsed/refractory (R/R) AML. This promising activity was also seen in high-risk groups, including patients with FLT3 mutations.1
Fludarabine, cytarabine, granulocyte-colony stimulating factor (G-CSF), and idarubicin (FLAG-IDA) is another commonly used therapy regimen for patients with AML, which produces good results in young, fit patients. However, results in the R/R setting are suboptimal. It is thought that combining this multidrug therapy with venetoclax, which increases apoptosis, may enhance leukemic cell death. At the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Curtis Lachowiez presented the interim results of a phase Ib/II study of venetoclax in combination with FLAG-IDA for the treatment of patients with newly diagnosed (ND) or R/R AML.2
Study design
The primary endpoints of the trial were to assess:
- The safety and tolerability of FLAG-IDA + venetoclax
- The maximum tolerated dose and dose-limiting toxicity
- Overall activity in ND AML and R/R AML
Secondary endpoints included assessment of:
- Response by revised International Working Group criteria
- Duration of response
- Overall survival (OS) and event-free survival (EFS)
- Biomarkers predictive of venetoclax sensitivity
Eligibility criteria
Patients were included in the trial if they had:
- ND or R/R AML
- High-risk myelodysplastic syndrome (≥ 10% blasts)
Exclusion criteria included:
- Acute promyelocytic leukemia
- Previous exposure to a Bcl-2 inhibitor
Induction and consolidation schedule
As five patients developed bacteremia during the phase Ib dosing, cytarabine was reduced from 2 g/m2 to 1.5 g/m2 and venetoclax was reduced from dosing on 1−21 days to only 1−14 days, as shown in the dosing schedule for the phase II induction/consolidation in Figure 1. G-CSF was given on the day before and the days of chemotherapy administration, followed by one dose of pegfilgrastim or biosimilar per 28-day cycle. During consolidation, idarubicin was given during two post-remission cycles e.g., C2 or C3 and C5 or C6 at the discretion of the physician.
Figure 1. Phase II induction/consolidation schedule2
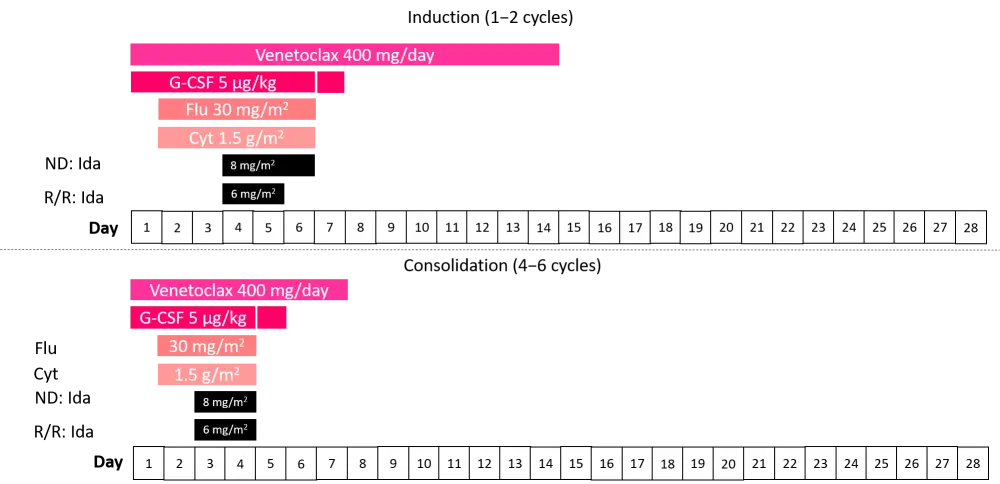
Cyt, cytarabine; Flu, fludarabine; G-CSF, granulocyte-colony stimulating factor; Ida, idarubicin; ND, newly diagnosed; R/R, relapsed/refractory.
Patient characteristics
The patient characteristics for this phase Ib/II trial (NCT03214562) are shown in Table 1. The patients were a young cohort with a median age of 46 years, and a small number of patients had extramedullary disease. The ND AML cases comprised 59% of patients with de novo AML and 41% with treatment-related or secondary AML. Out of all the patients enrolled, almost half were classified as having an adverse risk European Leukemia Network category.
Table 1. Patient baseline characteristics across the three study phases2
|
AML, acute myeloid leukemia; ELN, European Leukemia Network; ND, newly diagnosed; R/R, relapsed refractory. |
||||
|
Characteristic |
All |
Phase Ib R/R AML |
Phase IIa ND AML |
Phase IIb R/R AML |
|---|---|---|---|---|
|
Age |
46 (20−73) |
51 (20−65) |
45 (20−65) |
47 (22−66) |
|
Sex (male), % |
54 |
63 |
45 |
61 |
|
Median blast (%) |
44 (1−94) |
63 (6−94) |
41 (4−85) |
46 (1−86) |
|
Extramedullary AML |
4 |
— |
3 |
1 |
|
AML type, n |
|
|
|
|
|
De novo |
17 |
— |
17 |
— |
|
Secondary/treatment-related |
12 |
— |
12 |
— |
|
R/R |
39 |
16 |
— |
23 |
|
ELN risk group, % |
|
|
|
|
|
Favorable |
25 |
38 |
17 |
26 |
|
Intermediate |
26 |
13 |
45 |
13 |
|
Adverse |
49 |
50 |
38 |
61 |
Previous therapies
The therapies received by the patients with R/R AML are shown in Table 2. The patients included were predominantly in their first salvage treatment and had received a median of 1−2 lines of therapy previously. In total, 50% of the phase Ib cohort had received allogeneic hematopoietic stem cell transplantation (HSCT), whereas in the phase IIb cohort, less than a third had undergone this procedure.
Table 2. Previous treatments for patients with R/R AML2
|
allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; CR, complete response; R/R, relapsed/refractory. |
|||
|
Characteristic |
All R/R AML (n = 39) |
Phase Ib (n = 16) |
Phase IIb (n = 23) |
|---|---|---|---|
|
Median no. of prior therapies (range) |
1 (1−6) |
2 (1−6) |
1 (1−3) |
|
Prior allo-HSCT, % |
38 |
50 |
30 |
|
Median duration of prior CR, months (range) |
15.8 (2.3−70) |
15.1 (2.3−44) |
12.6 (2.7−70) |
|
Salvage #1, % |
69 |
50 |
83 |
|
Salvage #2, % |
15 |
19 |
13 |
|
Salvage ≥ #3, % |
15 |
31 |
4 |
Key points
Within the whole population, methylation was the most common type of aberration present as there were more IDH2 mutations in the intention to treat cohort. A significantly greater number of ND patients with AML presented with mutant IDH2 compared with in the R/R cohort (41% vs 15%, respectively; p = 0.025). In the ND cohort, there were significantly more patients in the diploid/intermediate group compared with the R/R group (76% vs 41%; p = 0.006). The latter had an even split between adverse and intermediate cytogenetic risk.
Only three patients transitioned to maintenance and these were in the phase Ib R/R AML cohort. There was no 30-day mortality in all groups. As presented in Table 3, in the phase II portion of the study, > 50% of the patients transitioned to HSCT. The median time to best response was similar across all groups at ~1 month. The median duration of response had not been reached in the ND or R/R phase II patients. Unsurprisingly, the median time to count recovery was greater in cycle two for all groups, as FLAG-IDA + venetoclax is known to cause count suppression.
Table 3. Treatment characteristics2
|
HSCT, hematopoietic stem cell transplant; NE, not estimable; NR, not reached. |
||||
|
Parameter |
All |
Phase IIa |
Phase Ib |
Phase IIb |
|---|---|---|---|---|
|
Median no. cycles (range) |
2 (1−6) |
2 (1−5) |
2 (1−6) |
2 (1−4) |
|
Median cycle length, days (n, range) |
|
|
|
|
|
C1 |
33 (48, 23−59) |
31 (26, 27−59) |
36 (9, 31−55) |
35 (13, 23−47) |
|
C2 |
41 (27, 26−91) |
42 (14, 27−60) |
47 (4, 26−91) |
37 (9, 26−90) |
|
C3 |
39 (15, 21−69) |
39 (10, 25−69) |
40 (2, 34−45) |
39 (3, 21−40) |
|
Transitioned to HSCT, % |
56 |
69 |
38 |
52 |
|
Median time to best response, days |
30 |
29 |
35 |
27 |
|
Median duration of response, months |
NR |
NR |
6 (3−NE) |
NR |
|
Median time to count recovery*, days |
|
|
|
|
|
C1 |
— |
31 |
37 |
37 |
|
C2 |
— |
46 |
62 |
38 |
|
C3 |
— |
41 |
40 |
40 |
A high overall response rate of 97% was achieved in patients with ND AML, and a 72% overall response rate was observed for patients with R/R AML. Composite CR (CRc) values were similar, with the highest rates achieved in patients with ND AML (90%) and a slightly lower rate (67%) observed for the R/R group. In the group of ND patients, 96% achieved measurable disease (MRD) negativity compared with 69% in the R/R group (Table 4). OS at 1-year was as high as 94% in the ND group, while EFS was not reached. In the R/R cohort an 11-month EFS was achieved in the phase IIB study, while OS was not reached. The 1-year OS in the phase IIB group was 68%.
Table 4. Response to treatment2
|
AML, acute myeloid leukemia; CR, complete response; CRh, CR with partial hematological recovery; CRi, CR with incomplete hematological recovery; MLFS, morphologic leukemia-free state; MRD, measurable disease response; ND, newly diagnosed; NE, not estimable; NR, not reached; OS, overall survival; R/R, relapsed/ refractory. |
|||||
|
Parameter |
All |
Phase IIa (ND AML) |
R/R AML |
Phase Ib |
Phase IIB |
|---|---|---|---|---|---|
|
Overall response, % |
82 |
97 |
72 |
75 |
70 |
|
Composite CR, % |
76 |
90 |
67 |
75 |
61 |
|
CR, n |
37 |
20 |
17 |
6 |
11 |
|
CRh, n |
10 |
5 |
5 |
2 |
3 |
|
CRi, n |
5 |
1 |
4 |
4 |
- |
|
MRD negative*, % |
83 |
96 |
69 |
58 |
79 |
|
MLFS, n |
4 |
2 |
2 |
|
2 |
|
No response, n |
12 |
1 |
11 |
4 |
7 |
|
Median EFS, months (range) |
— |
NR |
— |
6 (3−NE) |
11 (2−NE) |
|
Median OS, months (range) |
— |
NR |
— |
9 (4.9−NE) |
NR |
|
1-year OS, % |
— |
94 |
— |
38 |
68 |
In the R/R cohort, patients in salvage 1 (16 months [7−NE]) or 2 (14 months [11−NE]) had significantly improved OS compared with those in salvage 3 (4 months [3.8−NE]; p = 0.0033). The median OS and EFS of patients proceeding to transplant had not been reached. Of the patients with R/R AML who were treated, 46% transitioned to HSCT afterwards, demonstrating the suitability of FLAG-IDA + venetoclax as a bridging regimen. The 1-year OS post-HSCT was 78% in patients with R/R disease.
TP53 mutations were associated with poor outcomes despite treatment with FLAG-IDA + venetoclax. Out of all patients included in the trial, 15% were identified as having this genetic abnormality. Of the ten patients with mutated TP53, four patients did not respond to treatment. CRc was achieved in six patients, but four ultimately relapsed and the duration of response was only 3.3 months (1.9−not estimable) and five were deceased by the end of the trial.
Safety
The most common grade 3/4 adverse events were febrile neutropenia (ND, 48%; R/R, 51%), bacteriemia (ND, 21%; R/R, 46%), and pneumonia (28% in both groups). The most common complication during cycle one was infection and during cycle two it was myelosuppression.
Conclusion
FLAG-IDA + venetoclax was shown to be effective in treating patients with ND AML, achieving extremely high CRc and MRD-negative composite CR. Results were less favorable in the R/R AML population, however significant CRc rates and MRD-negative CRc were attained. The high proportion of patients transitioning to HSCT following treatment shows that this combination is a suitable bridging regimen. In addition, the combination of FLAG-IDA + venetoclax had an acceptable safety profile that was in line with previous studies. Analysis of patients with TP53-mutated AML in this study found that the prevalence of clones throughout treatment is dynamic and may persist in remission in some cases.
Expert Opinion
 Curtis Lachowiez
Curtis LachowiezReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

