All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Emerging mutations at relapse in patients with FLT3-mutated R/R AML who were treated with gilteritinib in the ADMIRAL trial
During an oral abstract session at the 25th European Hematology Association (EHA) Annual Congress, Catherine C. Smith presented abstract S147 on the emerging mutations at relapse in patients with FLT3-mutated relapsed/refractory (R/R) acute myeloid leukemia (AML) who received gilteritinib therapy in the phase III ADMIRAL trial.1
There is often a poor prognosis for patients with R/R AML as they are unlikely to respond to salvage chemotherapy. FLT3 internal tandem duplication (ITD) mutations are associated with increased risk of relapse and poor survival. With the emergence of FLT3-targeted therapies, the treatment paradigm for these patients has shifted. Gilteritinib, a highly selective, second-generation FLT3 inhibitor was approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) as a single agent based on the ADMIRAL phase III trial (NCT02421939). It has shown great promise in the R/R AML setting, demonstrating superior overall survival and deeper responses compared with salvage chemotherapy. However, secondary treatment-emergent mutations in the tyrosine kinase domain (TKD) can confer resistance to FLT3 inhibitor therapy. The aim of this study was to evaluate the emerging mutations in patients who relapsed on gilteritinib therapy in the ADMIRAL trial. 1
Methods1
- Blood/bone marrow samples were analyzed by next-generation sequencing using the ArcherDx Core Myeloid Panel, composed of 37 recurrently mutated genes in myeloid malignancies
- Full exon sequencing was performed for FLT3
- For all other genes, sequencing was conducted only for mutational hotspots
- Data were analyzed using the Archer Analysis software V5.1
- The variant allele frequency (VAF) cutoff for mutational positivity was 2.7% according to manufacturer specifications
Patient characteristics1
- Of the 247 patients assigned to the gilteritinib arm, 75 patients (30.5%) achieved a composite complete response (CRc) and relapsed, with a median time to relapse of 4.6 months (range, 1.3–20.3)
- 40 of the 75 patients (53.3%) had blood/bone marrow samples available at baseline and relapse for mutational evaluation
- 27 of the 40 patients (67.5%) had new mutations at relapse
- At baseline, 33 of the 40 patients (82%) had FLT3-ITD mutations, four (10%) had FLT3-TKD mutations, and three (8%) had both types of mutation
Results1
- The most common new gene mutations at relapse were the mitogen-activated protein kinases (Ras/MAPK) pathway genes (Figure 1A)
- NRAS was the most commonly mutated Ras/MAPK pathway gene, followed by PTPN11 and KRAS (Figure 1B)
Figure 1. New gene mutations at relapse1
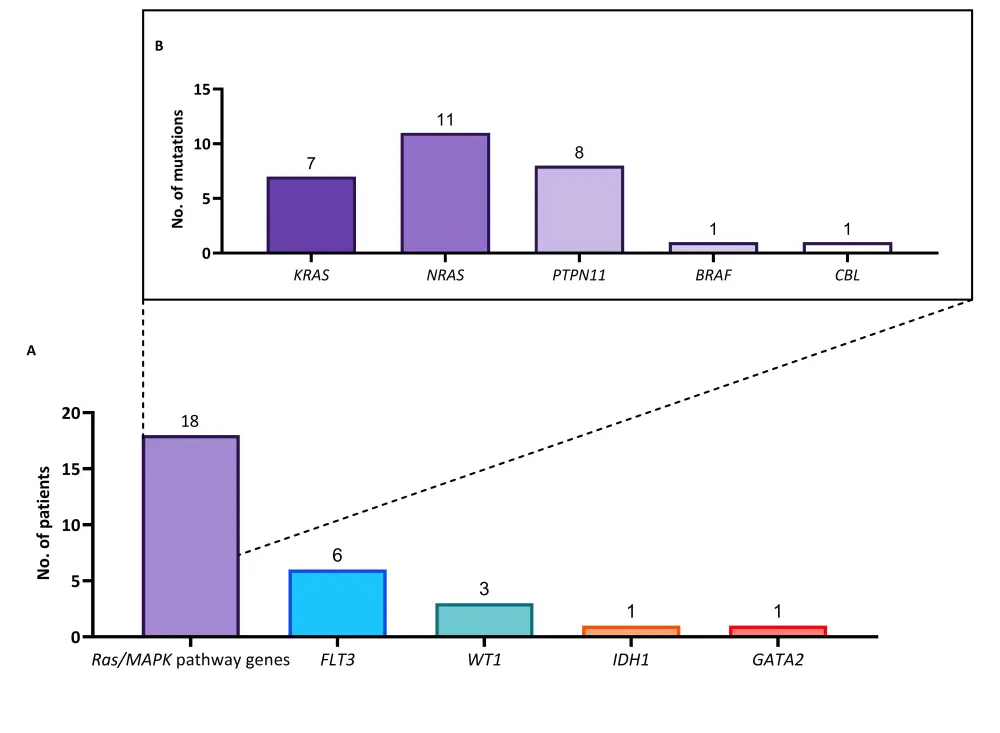
A The numbers of patients with each type of mutation. B The numbers of mutations of the Ras/MAPK pathway.
- Six patients developed new mutations within the FLT3 gene
- Five patients (83.3%) developed mutations in the F691L gate keeper residue
- Two patients (33.3%) developed mutations in the juxtamembrane domain (JMD) E598D
- One patient developed both F691L and JMD E598D mutations (VAF, 53% and 12%, respectively)
- One patient acquired both F691L and WT-1 mutations
- Two patients had Ras/MAPK mutations at baseline that persisted at relapse
- One patient had a KRAS G13D mutation with a VAF of 13% and 40% at baseline and relapse, respectively
- One patient had a NRAS G12D mutation with a VAF of 5% and 9% at baseline and relapse, respectively. This patient also acquired another NRAS G13D mutation at relapse with a VAF of 30%
- The acquisition of FLT3 F691L and Ras/MAPK pathway gene mutations were mutually exclusive
- Patients with Ras/MAPK pathway mutations at baseline had fewer mutations per patient compared with those that acquired new Ras/MAPK pathway mutations at relapse (Figure 2)
- Patients who acquired new Ras/MAPK pathway mutations at relapse had a median duration of CRc of 3.3 months (range, 0.9–13.8)
Figure 2. Comparison of Ras/MAPK gene mutations per patient at baseline and relapse1
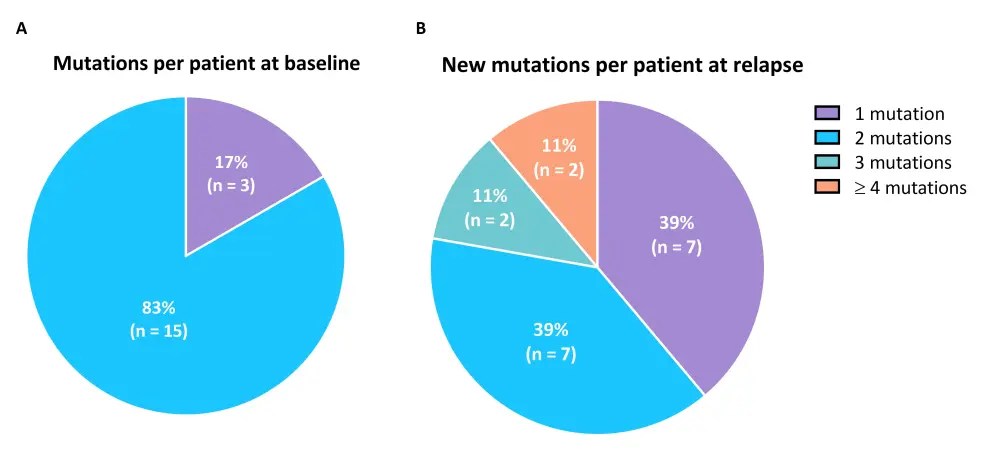
A The numbers of Ras/MAPK pathway gene mutations per patient at baseline. B The numbers of new Ras/MAPK pathway gene mutations per patient at relapse.
- 39% of patients who had a Ras/MAPK pathway gene mutation at baseline achieved a CRc with gilteritinib therapy
- Two of these patients (28.6%) went on to relapse
- 28% of patients who had a Ras/MAPK pathway gene mutation at baseline achieved a CR/CR with partial hematologic recovery (CRh) with gilteritinib therapy
Conclusion
The most common mutational events associated with gilteritinib resistance in patients with FLT3-mutated R/R AML in the ADMIRAL trial were mutations in Ras/MAPK pathway genes and FLT3 F691L mutations. The presence of Ras/MAPK pathway gene mutations at baseline did not preclude response to gilteritinib therapy as there were fewer mutations per patient at baseline than at relapse. The acquisition of Ras/MAPK pathway gene mutations at relapse is suggestive of reactivation of this pathway. The incidence of FLT3 F691L mutations was similar to those reported in patients with FLT3-mutated R/R AML who relapsed after gilteritinib treatment in the CHRYSALIS phase I/II study (NCT02014558).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

