All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
ELN-DAVID recommendations for MRD assessment in patients with AML
Do you know... Which of the following is not a measurable residual disease assessment technique?
Measurable residual disease (MRD) assessment is routinely used in patients with acute myeloid leukemia (AML) treated with intensive chemotherapy. However, its value in the context of lower-intensity regimens in older and unfit patients with AML remains uncertain. Due to a higher prevalence of preceding myelodysplastic syndromes and clonal hematopoiesis, particular consideration should be given to molecular MRD assessment in this population. The AML Hub has previously reported the impact of MRD status on outcomes in patients with AML treated with different therapies.
Here, we summarize recommendations from the European LeukemiaNet international working group for MRD Assessment and Validation in AML (ELN-DAVID) expert panel published by Ravandi et al.1 in American Journal of Hematology, which aimed to guide future research and clinical practice on MRD assessment in the management of patients with AML.
Methods
Despite the unavailability of sufficient data to make suggestions for the use of MRD in routine clinical practice, the panel developed recommendations to guide future research on MRD assessment using the Delphi poll to optimize consensus among members. The level of agreement score was reached using a Likert scale from 1 (completely disagree) to 5 (completely agree). Consensus was reached for all recommendations (Figure 1).
Recommendations
Figure 1. ELN-DAVID recommendations to guide further research on MRD assessment
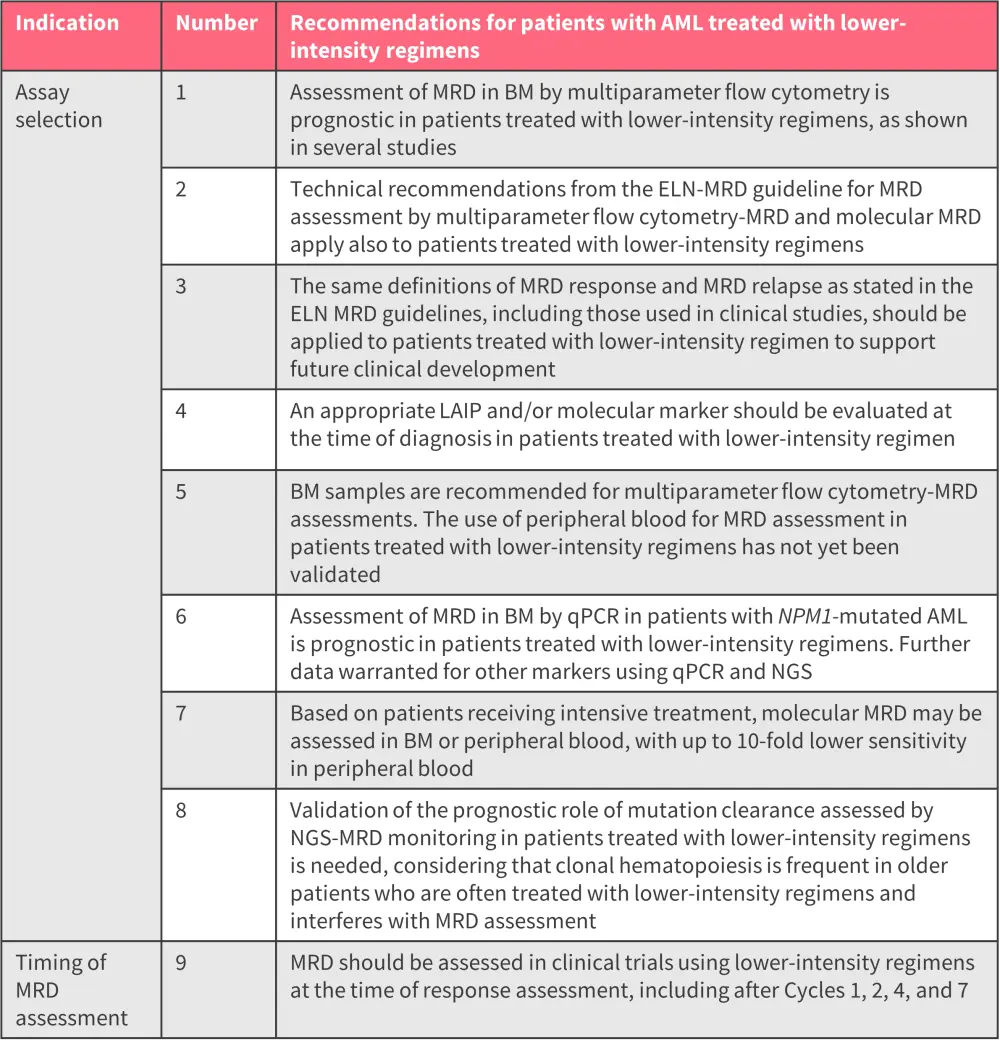
AML, acute myeloid leukemia; BM, bone marrow; ELN-DAVID, the European LeukemiaNet international working group for MRD Assessment and Validation in AML; LAIP, leukemia-associated immunophenotype; MRD, measurable residual disease; NGS, next-generation sequencing; qPCR, quantitative polymerase chain reaction.
*Adapted from Ravandi, et al.1
MRD measurement techniques
Techniques for assessing MRD include multiparameter flow cytometry, quantitative polymerase chain reaction, or next-generation sequencing. An MRD result that is below the suggested prognostic threshold is known as MRD negativity or undetectable MRD. Although this threshold has been defined for all three techniques, the prognostic effect of low MRD in patients receiving low-intensity treatment remains unknown and needs further investigation.
Recommendations for assay selection
The development of novel assays such as next-generation sequencing, digital droplet PCR, and multiplexed immunophenotyping has provided further scope for the detection and monitoring of MRD. However, until these assays are validated, technical recommendations and the definition of MRD response and MRD relapse recommended by the ELN-MRD guideline apply to patients with AML treated with lower-intensity regimens.
Molecular assessment of NPM1 MRD in bone marrow by quantitative polymerase chain reaction is prognostic in patients treated with venetoclax-based therapies; however, further data on other markers is needed. MRD in peripheral blood samples has shown prognostic value, with implications for MRD monitoring. Further studies are warranted to define the most appropriate thresholds in patients with NPM1-mutated AML who show prolonged survival with venetoclax-based therapies.
As traditional cytotoxic chemotherapy is mostly ineffective in patients with TP53-mutated AML, there is great interest in developing lower-intensity regimens using novel immune-based therapies. TP53 mutations are more frequent in older patients with AML, particularly in patients with prior myelodysplastic syndromes.
The prognostic value of mutation clearance by next-generation sequencing-MRD assessment needs further validation. However, the presence of clonal hematopoiesis in this population should be considered due to its ability to interfere with molecular MRD assessment.
Recommendations regarding the timing of MRD assessment
The panel recommended that MRD assessment using multiparameter flow cytometry should be undertaken after the first, second, fourth, and seventh cycles of therapy in clinical trials assessing hypomethylating agents or low-dose cytarabine with targeted therapies, in patients who achieve a morphological response. In later cycles, MRD may become undetectable, and the frequency of assessment may therefore be able to be reduced in routine clinical practice.
The optimal timing of MRD assessment may also be influenced by the type of molecular aberration and mode of drug action, alongside the potential of clonal selection and loss or gain of additional mutations.
Depth of MRD clearance achieved with lower-intensity treatment regimens
To use disease clearance as a tool for the prediction of clinical outcomes, the quality of responses achieved by lower-intensity regimens needs to be comparable to those with intensive chemotherapy. The ability to measure the depth of disease reduction is determined by assay sensitivity, which is impacted in patients treated with lower-intensity regimens by:
- bone marrow hypocellularity;
- lack of aberrant immunophenotypes; and
- presence of underlying clonal hematopoiesis.
Therefore, further investigation is needed regarding the depth of MRD clearance achieved by lower-intensity treatments and whether this is similar to that with intensive chemotherapy.
Conclusion
Rates of MRD responses are influenced by different regimens, patient populations, and assay selection. The ELN-DAVID expert panel’s recommendation provides a guide for MRD assessment in future clinical research assessing lower-intensity regimens in patients with AML. The recommendations also provide insight into the difficulties associated with MRD assessment in older, unfit patients with AML treated with novel and low-intensity regimens. The parameters for MRD monitoring to inform treatment decisions will be defined by further research using conventional and emerging MRD modalities.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


