All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
CPX-351 in combination with venetoclax: A phase II study in patients with R/R or newly diagnosed AML
Although there is a plethora of chemotherapeutic agents for the treatment of acute myeloid leukemia (AML), a number of patients relapse, suggesting additional treatments are still needed. CPX-351 is a liposomal formulation of cytarabine and daunorubicin in a 5:1 molar ratio, approved by the European Commission and the U.S. Food and Drug Administration (FDA) for the treatment of adults with newly diagnosed (ND) therapy-related AML or ND AML with myelodysplastic-related changes.
CPX-351 has shown significantly higher overall remission rates and better survival in patients with secondary AML compared with a standard 7+3 regimen.1 There is now interest in whether CPX-351 can safely be used in combination with existing chemotherapeutics to improve outcomes. At the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Tapan Kadia presented initial safety and efficacy results from a phase II study of CPX-351 with venetoclax in patients with AML (NCT03629171).2 This was identified as a practice-changing abstract by the AML Hub Steering Committee and their comments are included in a downloadable resource.
Study design
In the lead-in phase of the study, patients ≥18 years with relapsed/refractory (R/R) AML were eligible. Once safety was established, two expansion cohorts were included. Cohort A included adult patients with R/R AML, whilst cohort B was for patients aged 18–65 years who were ND and had not received any prior treatment for AML.
The primary endpoint was efficacy assessed by complete remission and complete remission with incomplete hematologic recovery. Secondary endpoints were safety, and overall and relapse-free survival.
Induction and consolidation schedule
In the induction phase, intravenous CPX-351 at a dose of daunorubicin 44 mg/m2 and cytarabine 100 mg/m2 was given on Days 1, 3, and 5. During the consolidation cycles, intravenous CPX-351 at a dose of daunorubicin 29 mg/m2 and cytarabine 65 mg/m2 was administered on Days 1 and 3. In the safety lead-in cohort, oral venetoclax was started at 300 mg daily on Days 2–21 during induction and consolidation. Reduced doses of venetoclax were permitted for patients concomitantly taking strong or moderate CYP3A inhibitors. If, on Day 14, the bone marrow was hypocellular then venetoclax was interrupted. Due to the development of dose-limiting cytopenias in three patients within the lead-in phase, the regimen of venetoclax 300 mg was reduced from 20 to 7 days (given on Days 2–8).
Patient characteristics
Overall, 20 patients have thus far been treated in the study, of which 18 have outcome data available. Of these, 12 patients were in the safety lead-in cohort, five in cohort A, and one in cohort B. Patient characteristics are shown in Table 1. At baseline, the TP53 mutation was common, present in 38% of patients.
Table 1. Patient characteristics for all cohorts, including the safety lead-in and expansion cohorts A and B*
|
AML, acute myeloid leukemia; ND, newly diagnosed; R/R, relapsed/refractory. |
|
|
Characteristic |
All cohorts |
|---|---|
|
Median age, years (range) |
51 (29–71) |
|
Diagnosis, % |
|
|
R/R AML |
94 |
|
ND secondary AML |
6 |
|
Prior therapies, median (range) |
2 (1–8) |
|
Prior venetoclax, n (%) |
7 (41) |
|
Cytogenetics, % |
|
|
Diploid, -Y |
15 |
|
Adverse |
45 |
|
Intermediate, non-diploid |
25 |
|
Favorable |
5 |
|
Insufficient |
10 |
|
Median peripheral blood blast, % (range) |
27 (4–96) |
Key results
Efficacy
Response and survival rates are shown in Table 2 and Figure 1. Across all cohorts, an overall response rate of 44% was achieved. For the eight patients who responded to CPX-351 plus venetoclax, there was an estimated 1-year relapse-free survival of 86%. Furthermore, response to CPX-351 plus venetoclax was associated with better survival at 6 months (86%) compared with non-responders (18%). There was also a trend towards improved survival in patients with no prior venetoclax exposure (median, survival not reached; n = 10) compared with those with prior venetoclax exposure (median, 5 months; n = 6; p = 0.43).
Table 2. Response to CPX-351 and venetoclax combination treatment across all cohorts*
|
CR, complete response; CRi, CR with incomplete hematologic recovery; HSCT, hematopoietic stem cell transplant; MFLS, morphologic leukemia-free state; NR, not reached; ORR, overall response rate; OS, overall survival; RFS, relapse-free survival. |
|
|
|
All cohorts |
|---|---|
|
Median number of cycles (range) |
1 (1–2) |
|
Median time to count recovery, days (range) |
41 (23–60) |
|
Response, n (%) |
|
|
ORR |
8 (44) |
|
CR |
1 (6) |
|
CRi |
6 (33) |
|
MLFS |
1 (6) |
|
Transitioned to HSCT, n (%) |
7 (88) |
|
Mortality, % |
|
|
At 4 weeks |
10 |
|
At 8 weeks |
20 |
|
Median OS, months |
6.1 |
|
Responder |
NR |
|
Non-responder |
1.7 |
|
No prior venetoclax |
NR |
|
Prior venetoclax |
5 |
|
Median RFS, months |
|
|
Responders |
NR |
Figure 1. Survival rates at 6 months and 1 year, stratified by response to CPX-351 plus venetoclax and prior venetoclax exposure*
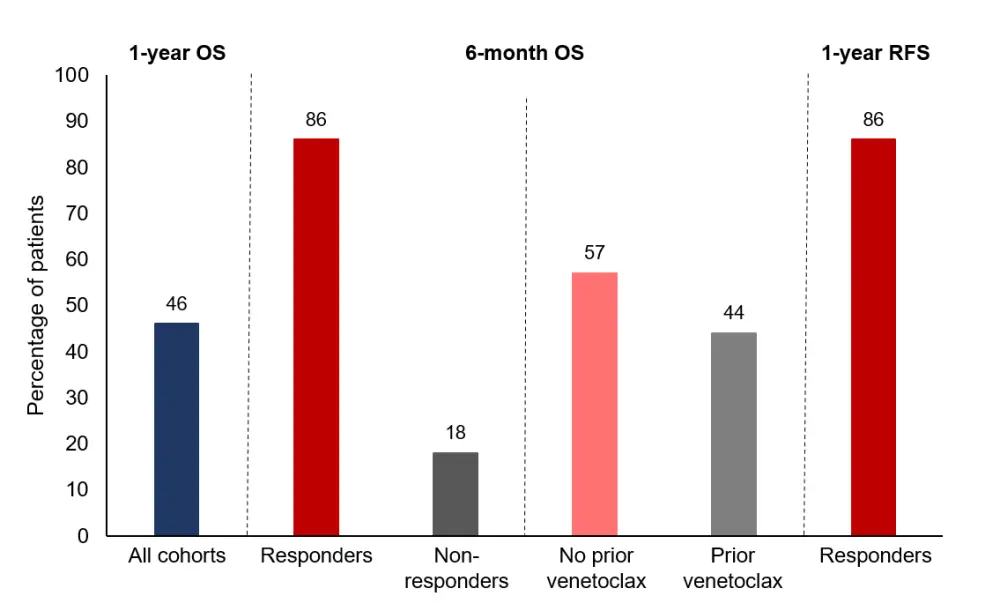
OS, overall survival; RFS, relapse-free survival.
*Adapted from Kadia et al.2
Safety
The combination dose of CPX-351 with 20 days of venetoclax resulted in dose-limiting prolonged myelosuppression; however, tolerability was improved with adjustment of the regimen to CPX-351 plus 7 days of venetoclax. The most common Grade 3/4 adverse events were infection (n=7), nausea (n = 4), and pneumonia (n = 3).
Conclusion
In this phase II study, a combination of the liposomal cytarabine-daunorubicin formulation CPX-351 plus venetoclax was assessed for safety and efficacy in patients with R/R AML and expanded to include those with ND AML. The authors concluded that the safety profile was acceptable and clinical responses were promising for the CPX-351 plus 7 days of venetoclax regimen, particularly in patients who had not previously received venetoclax. The majority of responders were able to proceed to hematopoietic stem cell transplant. Recruitment is continuing within the expansion cohorts.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

