All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
ADMIRAL trial: Patient-reported outcomes in patients with FLT3-mutated relapsed/refractory AML
Do you know... In patients with relapsed/refractory FLT3-mutated AML receiving gilteritinib, which of the following Functional Assessment of Cancer Therapy-Leukemia subscale is not likely to be associated with an inferior overall survival (OS)?
Patient-reported outcomes (PROs) are an important metric for the assessment of the impact of treatment on the health status of patients with acute myeloid leukemia (AML).1 Routine evaluation of PROs can help inform treatment decision-making and improve prognosis by identifying factors associated with survival.1 The phase III ADMIRAL trial (NCT02421939) previously covered by the AML Hub investigated the efficacy of gilteritinib, a highly selective oral FLT3 inhibitor versus salvage chemotherapy (SC) in patients with relapsed/refractory (R/R) AML with FLT3 mutations.
While PROs were evaluated in the ADMIRAL trial, the data collection for PROs was ongoing during the time of the primary analysis; therefore, not included in the previous publication.2 Ritchie et al.1 recently published the PROs analysis from the ADMIRAL trial in Leukemia & Lymphoma which we are pleased to summarize below.
Study design and patient characteristics
The open-label, multicenter, phase III ADMIRAL trial included patients aged ≥18 years with R/R FLT3-mutated AML. Eligible patients received either gilteritinib or SC in a continuous 28-day cycle. The primary endpoints of the ADMIRAL trial were overall survival (OS) and complete remission (CR) and CR with partial hematologic recovery (CRh), with patient-reported fatigue as a secondary endpoint. The PRO instruments assessed included:
- Brief Fatigue Inventory (BFI)
- Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu)
- Functional Assessment of Chronic Illness Therapy-Dyspnea (FACIT-Dys)
- EuroQoL 5-Dimension 5-Level (EQ-5D-5L)
- Leukemia-specific symptoms including dizziness and mouth sores
PROs were assessed at baseline, on Day 1 of each treatment cycle, and at the end-of-treatment (EOT) visit. Additionally, BFI was assessed on Day 8 and Day 15 of Cycle 1, and Day 1 and Day 15 of Cycle 2. At each cycle, post-baseline item scores and domain scores were categorized as either improvement, no change, or deterioration relative to baseline scores based on the change threshold. This was defined by published values indicating a clinically meaningful change or on a distribution-based method in the absence of a published value. In total, 246 patients received gilteritinib and 109 patients received salvage chemotherapy.
Key findings
Changes in PROs from baseline
Baseline fatigue levels were low and remained generally low in both treatment arms (Table 1). A higher proportion of patients in the gilteritinib arm had a ≥3 point improvement in the BFI worst fatigue item rating versus patients in the SC arm on Day 8 (16.2% vs 6.8%) and Day 15 (24.4% vs 6.8%) of Cycle 1. Patients in the gilteritinib arm also had lower rates of ≥3 point deterioration in the BFI severity item of worst fatigue compared with patients in the SC arm on Day 8 (11.7% vs 27.4%) and Day 15 (14.2% vs 35.6%) of Cycle 1.
Table 1. Change in BFI scores*
|
BFI, Brief Fatigue Inventory; GIL, gilteritinib; mEOT, modified end-of-treatment; SC, salvage chemotherapy; SD, standard deviation. |
||||||||
|
BFI domain score |
Baseline |
Cycle 1, Day 8 |
Cycle 2, Day 2 |
mEOT |
||||
|---|---|---|---|---|---|---|---|---|
|
GIL |
SC |
GIL |
SC |
GIL |
SC |
GIL |
SC |
|
|
Total |
|
|
|
|
|
|
|
|
|
Mean |
3.0 |
2.7 |
2.5 |
3.7 |
2.9 |
2.5 |
4.0 |
4.0 |
|
Median |
2.6 |
2.0 |
2.1 |
3.6 |
2.3 |
2.2 |
3.8 |
3.4 |
|
Severity |
|
|
|
|
|
|
|
|
|
Mean |
3.5 |
3.2 |
3.0 |
4.2 |
3.3 |
3.1 |
4.4 |
4.4 |
|
Median (range) |
3.0 |
3.0 |
2.7 |
4.0 |
3.0 |
2.3 |
4.3 |
4.7 |
|
Interference |
|
|
|
|
|
|
|
|
|
Mean |
2.8 |
2.5 |
2.3 |
3.4 |
2.7 |
2.2 |
3.8 |
3.8 |
|
Median |
2.2 |
1.7 |
1.5 |
3.3 |
1.8 |
1.5 |
3.6 |
3.3 |
Mean FACT-Leu total scores were similar between both treatment arms at baseline (Table 2). FACT-Leu subscale scores remained stable throughout, with a modest improvement by modified EOT (mEOT) assessment in the gilteritinib arm.
Table 2. Change in FACT-Leu scores*
|
EWB, emotional well-being; FACT-Leu, Functional Assessment of Cancer Therapy-Leukemia; FWB, functional well-being; GIL, gilteritinib; Leu-S, leukemia subscale; mEOT, modified end-of-treatment; PWB, physical well-being; SC, salvage chemotherapy; SD, standard deviation; SWB, social/family well-being; TOI, trial outcome index. |
||||||
|
FACT-Leu domain scores |
Baseline |
Cycle 2, Day 1 |
mEOT |
|||
|---|---|---|---|---|---|---|
|
GIL arm |
SC arm |
GIL arm |
SC arm |
GIL arm |
SC arm |
|
|
Total |
|
|
|
|
|
|
|
Mean |
122.1 |
118.5 |
123.8 |
124.2 |
116.0 |
113.8 |
|
Median |
125.8 |
123.0 |
128.0 |
123.0 |
118.0 |
115.0 |
|
TOI |
|
|
|
|
|
|
|
Mean |
83.6 |
81.1 |
85.2 |
85.3 |
79.0 |
76.5 |
|
Median |
85.0 |
83.0 |
85.0 |
84.0 |
83.0 |
79.0 |
|
General |
|
|
|
|
|
|
|
Mean |
74.6 |
72.5 |
75.9 |
74.7 |
70.7 |
69.5 |
|
Median |
76.0 |
73.0 |
78.4 |
79.0 |
69.7 |
68.0 |
|
PWB |
|
|
|
|
|
|
|
Mean |
21.4 |
21.3 |
22.4 |
22.7 |
19.9 |
20.1 |
|
Median |
23.0 |
23.0 |
24.5 |
23.0 |
22.0 |
22.0 |
|
EWB |
|
|
|
|
|
|
|
Mean |
16.2 |
15.9 |
17.2 |
17.3 |
16.0 |
15.6 |
|
Median |
17.0 |
17.0 |
18.0 |
18.0 |
17.0 |
17.0 |
|
FWB |
|
|
|
|
|
|
|
Mean |
14.7 |
13.8 |
15.0 |
13.1 |
16.0 |
12.2 |
|
Median |
14.0 |
13.0 |
14.0 |
13.0 |
13.0 |
11.0 |
|
SWB |
|
|
|
|
|
|
|
Mean |
22.3 |
21.5 |
21.4 |
21.6 |
21.0 |
21.6 |
|
Median |
24.0 |
23.3 |
23.3 |
21.0 |
22.0 |
22.0 |
|
Leu-S |
|
|
|
|
|
|
|
Mean |
47.6 |
46.0 |
47.9 |
49.5 |
45.2 |
44.3 |
|
Median |
49.0 |
47.0 |
50.0 |
54.0 |
48.0 |
46.0 |
Mean dyspnea scores and functional limitation scores were similar between the two arms at baseline (Table 3). The number of patients with no change or improvement in dyspnea or functional limitation scores on Day 1 of Cycle 2 or mEOT were similar in both arms.
Table 3. Change in FACIT-Dys scores*
|
FACIT-Dys, Functional Assessment of Chronic Illness Therapy-Dyspnea; GIL, gilteritinib; mEOT, modified end-of-treatment; SC, salvage chemotherapy; SD, standard deviation. |
||||||
|
FACIT-Dys domain score |
Baseline |
Cycle 2, Day 1 |
mEOT |
|||
|---|---|---|---|---|---|---|
|
GIL arm |
SC arm |
GIL arm |
SC arm |
GIL arm |
SC arm |
|
|
Dyspnea |
|
|
|
|
|
|
|
Mean |
7.4 |
7.1 |
6.1 |
7.6 |
9.0 |
9.5 |
|
Median |
5.0 |
4.0 |
4.0 |
4.5 |
6.0 |
6.5 |
|
Functional limitation |
|
|
|
|
|
|
|
Mean |
6.5 |
5.9 |
4.9 |
5.5 |
7.6 |
7.8 |
|
Median |
3.2 |
3.0 |
3.0 |
4.5 |
5.0 |
4.0 |
The proportion of patients with mild symptoms of dizziness and mouth sores was similar between both arms (Table 4). While more patients in the gilteritinib arm experienced an increase in dizziness by the mEOT assessment (50% vs 10%), overall, most patients in both arms had no change in dizziness (62.9%) and mouth sores (73.3%).
Table 4. Change in Leukemia-specific symptom scores*
|
GIL, gilteritinib; mEOT, modified end-of-treatment; SC, salvage chemotherapy; SD, standard deviation. |
||||||
|
Leukemia-specific symptom score |
Baseline |
Cycle 2, Day 1 |
mEOT |
|||
|---|---|---|---|---|---|---|
|
GIL arm |
SC arm |
GIL arm |
SC arm |
GIL arm |
SC arm |
|
|
Dizziness |
|
|
|
|
|
|
|
Mean |
0.4 |
0.5 |
0.6 |
0.4 |
0.8 |
0.6 |
|
Median |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
|
Mouth sores |
|
|
|
|
|
|
|
Mean |
0.3 |
0.3 |
0.4 |
0.1 |
0.5 |
0.5 |
|
Median |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
Mean EQ-5D-5L visual analog scale (VAS) values at baseline were similar between both arms (Table 5). Mean EQ-5D-5L VAS scores were stable during Cycle 1 and Cycle 2 in both arms and the rates of no change or improvement in scores were similar.
Table 5. Change in EQ-5D-5L VAS scores*
|
EQ-5D-5L, EuroQoL 5-Dimension 5-level; GIL, gilteritinib arm; mEOT, modified end-of-treatment; SC, salvage chemotherapy arm; SD, standard deviation; VAS, Visual Analog Scale. |
||||||
|
EQ-5D-5L |
Baseline |
Cycle 2, Day 1 |
mEOT |
|||
|---|---|---|---|---|---|---|
|
GIL arm |
SC arm |
GIL arm |
SC arm |
GIL arm |
SC arm |
|
|
VAS score |
|
|
|
|
|
|
|
Mean |
63.9 |
62.9 |
65.5 |
67.2 |
62.1 |
61.9 |
|
Median |
70.0 |
69.0 |
70.0 |
67.0 |
69.0 |
62.5 |
PROs and survival outcomes
Clinically meaningful deterioration of BFI, FACT-Leu, FACIT-Dys, and EQ-5D-5L scores were associated with inferior OS in both arms (Figure 1). Multivariable analyses revealed that OS and event-free survival remained significantly associated with PROs after adjusting for baseline prognostic factors. In the gilteritinib arm, deteriorating BFI, FACIT-Dys, EQ-5D-5L, and FACT-Leu scores excluding social/family well-being were associated with inferior OS (Figure 1). Deteriorating BFI, FACIT-Dys, and EQ-5D-5L scores were also associated with relapse/disease progression (BFI, p < 0.05; FACIT-Dys, p < 0.005; EQ-5D-5L, p < 0.05).
Figure 1. Association of PROs and overall survival in A ITT population and B gilteritinib arm*
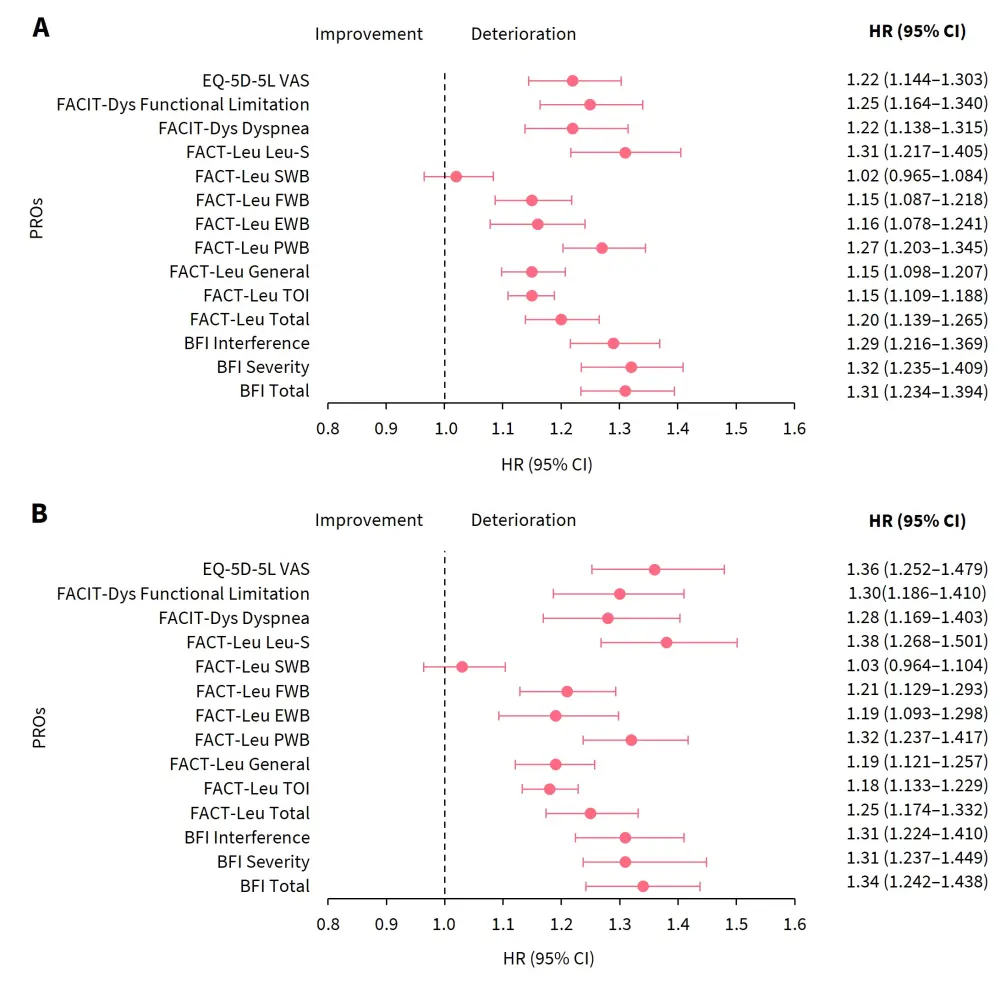
BFI, Brief Fatigue Inventory; CI, confidence interval; EWB, emotional well-being; EQ-5D-5L, EuroQoL 5-Dimension 5-level; FACIT-Dys, Functional Assessment of Chronic Illness Therapy-Dyspnea; FACT-Leu, Functional Assessment of Cancer Therapy-Leukemia; FWB, functional well-being; HR, hazard ratio; ITT, intention to treat; Leu-S, leukemia subscale; PRO, patient-reported outcome; PWB, physical well-being; SWB, social/family well-being; TOI, trial outcome index; VAS, Visual Analog Scale.
*Adapted from Ritchie, et al.1
PROs and transfusion status
At baseline, 197 patients in the gilteritinib arm were transfusion-dependent, of which 68 patients (34.5%) became transfusion-independent (TI), while 29 (59.2%) of the 49 patients who were TI at baseline continued to be TI. Increased BFI scores were observed in patients who remained or became transfusion-dependent. Higher baseline FACT-Leu scores were associated with TI status at baseline.
PROs and transfusion status
In the gilteritinib arm, 63 out of 247 patients underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT). These patients showed a higher rate of CR and CRh versus those who did not undergo allo-HSCT (CR, 39.7% vs 14.7%; CRh, 15.9% vs 12%). Patients undergoing allo-HSCT were more likely to either maintain or show an improvement in PRO across most of BFI, FACT-Leu, and EQ-5D-5L subscales.
PROs and hospitalization
Any episode of first hospitalization was associated with deteriorating PRO scores except for FACT-Leu social/family well-being, emotional well-being, and functional well-being subscales. Hospitalization in the intensive care unit occurred in a small number of patients and was associated with the worsening of most PRO scores.
Conclusion
This study showed that gilteritinib may improve patient-reported fatigue compared with SC in Cycle 1. Improved PROs were also associated with better survival outcomes in the gilteritinib arm. PROs can be an important factor in the therapy selection and shared decision-making to improve the clinical outcomes in patients with AML. This study was limited by the descriptive nature of the PROs and the lack of comparisons between the two treatment arms beyond Cycle 2 due to early discontinuation in the SC arm. Further studies are warranted to determine the prognostic value of PROs and the impact of PROs on hospitalization. PROs should also be investigated in patients with R/R FLT3-mutated AML who are receiving FLT3 inhibitors and are ineligible for allo-HSCT.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



