All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know AML.
The aml Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the aml Hub cannot guarantee the accuracy of translated content. The aml and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The AML Hub is an independent medical education platform, sponsored by Daiichi Sankyo, Johnson & Johnson, Syndax, Thermo Fisher Scientific, Kura Oncology, AbbVie, and has been supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group.
Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View AML content recommended for you
Addition of midostaurin to frontline 7+3: A case report
During the 2nd How to Diagnose and Treat Acute Leukaemia conference of the European School of Haematology (ESH), our AML Hub Executive Steering Committee member, Jorge Sierra, delivered an oral presentation on the addition of midostaurin to frontline cytarabine plus the anthracycline, idarubicin (7+3) for the treatment of acute myeloid leukemia (AML), with a focus on a case report.1 This is the first in a series of case reports about the addition of a third agent to frontline 7+3.
Patient details1
- 56-year-old male
- Past medical history: dyslipidemia treated with pravastatin
- November 2016 presentation: fatigue, fever, periodontal infection that was not responding to antibiotics
- Low hemoglobin and platelet count (73 g/L and 34 × 109/L, respectively)
- Mild leukocytosis (white blood cell [WBC] count of 12.5 × 109/L; 90% blasts)
- Bone marrow aspirate: 93% blasts
- Cytogenetics:
- 47,XY,+8[11]/46,XY[9]; trisomy 8 present in 11 metaphases
- NPM1 mutation (56.734 copies)
- High ratio of FLT3-internal tandem duplication (ITD) mutation in the juxtamembrane domain (ratio: 0.9)
- Negative for mutations in IDH1/2, CEBPA, AML-1/ETO, and CBFB/MYH11 rearrangements, and MLL tandem duplication
Diagnosis1
- AML with NPM1 mutation and FLT3-ITD high ratio
- Intermediate risk, according to European LeukemiaNet (ELN) criteria (2017)2
|
Approximately a third of patients with AML have FLT3-ITD or tyrosine kinase domain (TKD) mutations, which lead to constitutive activation of FLT3, thus increasing proliferation and driving resistance to chemotherapy and apoptosis. FLT3 mutations are associated with a high WBC count and relapse rate. Midostaurin is Type I FLT3 inhibitor (non-specific and effective against both TKD and ITD mutations).1 |
|
During the 61st American Society of Hematology (ASH) Meeting & Exposition in Orlando, US, 2019, Jorge Sierra presented data (previously reported on the AML Hub) that demonstrated patients with intermediate cytogenetics (according to the Medical Research Council classification), with an NPM1 mutation and low allelic ratio of FLT3-ITD, had a similar outcome compared with those who had an NPM1 mutation without FLT3 mutation (74 ± 9% and 66 ± 18%, respectively). Moreover, those with an NPM1 mutation and high FLT3-ITD allelic ratio had a very poor prognosis (26 ± 16%).1 |
Management plan
The patient’s management plan is demonstrated in Figure 1 and discussed in more detail below.
Figure 1. Treatment management plan1
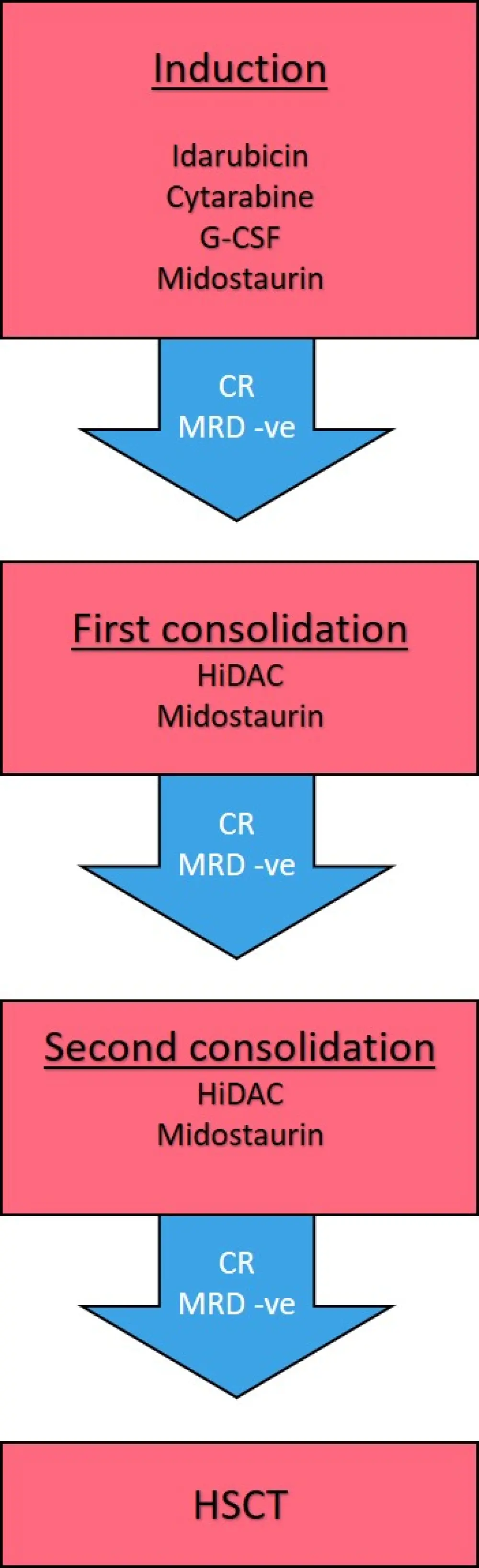
Blue arrows indicate response to treatment.
Minimal residual disease was measured by flow cytometry and NPM1 copy number.
CR, complete remission; G-CSF, granulocyte-colony stimulating factor; MRD -ve, minimal residual disease negativity; HiDAC, high dose cytarabine; HSCT, hematopoietic stem cell transplant
Induction
- Idarubicin (12 mg/m2 on Days 1–3), cytarabine (200 mg/m2 on Days 1–7), granulocyte-colony stimulating factor (if WBC lower than 30 × 109/L)1,3
- Compassionate use midostaurin was also administered as the patient had a FLT3-ITD mutation (50 mg on Days 21–34; treatment delayed due to compassionate use application)
|
This treatment regimen was based on the AML-12 trial of the Spanish CETLAM group (previously reported on the AML Hub), where 77% of patients achieved a complete response.3 |
|
ELN 2017 treatment approach for patients ≤ 60–65 years stated an induction therapy of 7+3 (cytarabine + anthracycline), with high doses of cytarabine generally considered as the best treatment option for those not responding to the first cycle of 7+3. Those with newly diagnosed AML and activating FLT3 mutations were also considered to receive additional therapy with midostaurin following chemotherapy. (These guidelines were accepted before the approval of midostaurin by the U.S. Food and Drug Administration [FDA] and European Medicines Agency [EMA]).1,2 |
|
Current National Comprehensive Cancer Network (NCCN) guidelines V3.2020 state the use of standard dose cytarabine 200 mg/m2 continuous infusion for 7 days, with idarubicin 60 mg/m2 for 3 days, and oral midostaurin at 50 mg every 12 hours from Days 8–21 for those with FLT3-mutated AML.1 |
|
The use of midostaurin in first line treatment was based on the phase III RATIFY study (previously reported by the AML Hub), where there was a 24.3% lower risk of death in the midostaurin group than in the placebo group.1,4 |
The patient responded to induction therapy, with a complete remission (CR) with minimal residual disease (MRD) negativity by flow cytometry and NPM1 copy number.
The patient then received first consolidation with high dose cytarabine (HiDAC; 3 g/m2) plus midostaurin on Days 8–21, and remained in CR with MRD negativity. He was given a second consolidation with the same treatment, and subsequently remained MRD-negative and in CR. As the patient was in first complete remission (CR1), he was eligible for a hematopoietic stem cell transplantation (HSCT).
|
The ELN 2017 guidelines state that consolidation treatment for patients with intermediate risk genetics should be allogeneic HSCT, or intermediate dose cytarabine (IDAC), or HiDAC with autologous HSCT. Allogeneic HSCT in CR1 is the most favorable treatment option.1 |
HSCT1
- Admitted in May 2017 for HLA-identical unrelated donor HSCT
- Conditioning: busulfan Day -6 to Day -4; fludarabine Day -6 to Day -2
- Graft-versus-host disease (GvHD) prophylaxis: sirolimus from Day -5; tacrolimus from Day -3
However, the donor experienced a splenic rupture, and therefore peripheral blood stem cell mobilization and collection were cancelled.
The patient received a haploidentical HSCT from his daughter.
- Conditioning: thiotepa (2.5 mg/kg per day on Days -7 and -6; fludarabine (50 mg/m2 per day on Days -5 to -3), busulfan (1 mg/kg per day on Days -5 to -3)
- Infused cells: 5.0 × 106 CD34+ per kg, 7.09 × 108 cell number per kg, 3.22 × 106 CD3+ per kg
- At infusion, the patient’s blood test showed hemoglobin 118 g/L, platelets 30 × 109/L, WBC 1 × 109/L (0.97 × 109/L neutrophils)
- GvHD prophylaxis: cyclophosphamide (50 mg/kg per day on Days 3 and 4); tacrolimus (1 mg/kg continuous infusion from Day 5)
- Patient recovered quickly (absolute neutrophil count recovery at Day 20)
- At Day 27, the patient was in CR and MRD-negative
Post-transplant evolution
- At Day 64, the patient was MRD-negative, had 1% blasts, 0.01 NPM1 copies, and 100% donor chimerism
- This situation remained at Day 97 and at 7 months posttransplant
|
The phase II DE02T trial (AMLSG 16-10) (previously reported on the AML Hub) assessed the effect of midostaurin in combination with HiDAC with subsequent allogeneic HSCT, and demonstrated that midostaurin significantly improved event free survival (HR, 0.58; 95% CI, 0.48–0.70; P < 0.001).5 |
|
The RADIUS phase II study (previously reported on the AML Hub) examined the use of a midostaurin combination with standard of care (SOC) as maintenance after allogeneic HSCT. The study showed no significant improvement with midostaurin posttransplant, although there was a 54% relative reduction in the risk of relapse compared with SOC.1 |
Conclusion
Although FLT3-ITD usually confers poor prognosis, midostaurin improves overall survival when added to intensive front-line chemotherapy followed by maintenance. Jorge Sierra concluded that the best treatment strategy in fit young patients with FLT3-ITD-mutated AML is chemotherapy plus midostaurin, followed by allogeneic transplantation in CR1. This is well demonstrated by this case study, and presently the patient remains in a perfect general status (Eastern Cooperative Oncology Group status 0), with continuous CR and MRD-negativity at 3 years posttransplant, and no GvHD.
For more information on how MRD impacts the outcome of HSCT in patients with AML, follow our AML Hub Satellite Symposium. The event will take place on August 30, 2020, at 8:30 A.M. CEST, during the 46th Annual Meeting of the European Society for Blood and Marrow Transplantation, and will feature five international experts, Gert Ossenkoppele, Jacqueline Cloos, Christian Thiede, Adriano Venditti, and Charles Craddock.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

